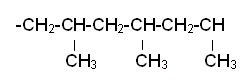Polymers
- A polymer is a long molecule made up of lots of repeating units called monomers.
- If all the monomers in a polymer are the same, we can represent them with a letter “A”; A-A polymer forms:
A + A + A + A -- 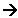 -- A-A-A-A –
-- A-A-A-A –
- Polythene and pvc are examples of A-A polymers.
- If a polymer is made up of two different monomers, we can get an A-B polymer:
A + B + A + B -- 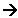 -- A-B-A-B –
-- A-B-A-B –
- Nylon-6,6 and polyesters are examples of this type of polymer.
- Writing out a long hand polymer would be time consuming, therefore a short-hand way of writing out the formula of a polymer has been developed.
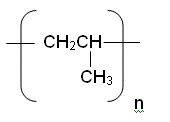
- Poly(propene) can be written out like:
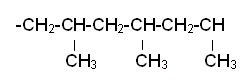
- However, it is much easier to represent poly(propene) with:
Properties of Polymers
- There are six factors that the properties of a polymer are dependent upon:
Chain Length- the longer the chain, the stronger the polymer. Tensile strength can be described as the amount of force that needs to be applied before the chain breaks.
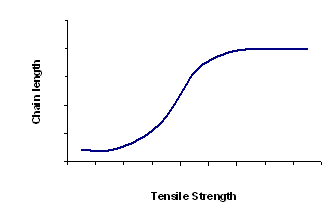 Tensile strength increases with chain length, because:
Tensile strength increases with chain length, because:
- Longer chains become more entangled together.
- When chains are longer they have more points of contact with chains of neighbouring polymers. There are more intermolecular forces to hold the chains together.
- Side groups on polymer chain- The more polar the side groups on the chain (e.g. Br or Cl), the stronger the attraction between the neighbouring chains; thus the stronger the polymer.
- Branching- The more unbranched the chain is, the tighter the packing, and so the stronger the attraction between the chains. This makes the polymer stronger.
- Chain flexibility- The more rigid the chain, the stronger the polymer.
- Cross-linking- The more extensive the cross-linking, the harder it is to melt the polymer.
- Stereoregularity- The more regular the orientation of the side groups, the closer the packing, therefore the stronger the polymer.
- Polymer properties vary widely.
- Elastomers are polymers that are soft and springy; when they are deformed they go back to their original shape. Rubber is an example of an elastomer.
- Plastics are polymers which undergo permanent or “plastic” deformation; they don’t go back to their original shape after being deformed. Polythene is an example of a plastic.
- Fibres are stronger polymers that don’t deform easily. Fibres are useful in clothing, and examples of them are polyester and nylon.
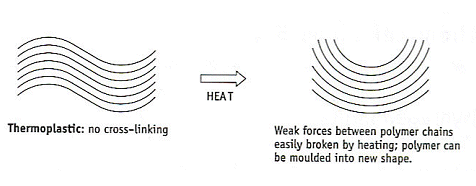
Thermoplastics
- These are made from polymers without cross-linking between their chains.
- The intermolecular forces between the chains are relatively weak (compared to thermosets with covalent corss-links).
- The attractive forces in the thermoplastics can be broken down by warming.
- The chains are able to move over each other and the polymer can be deformed.
- On cooling, the weak forces between the polymer reform and the thermoplastic holds its new shape.
- Examples of thermoplastics are polythene and nylon.
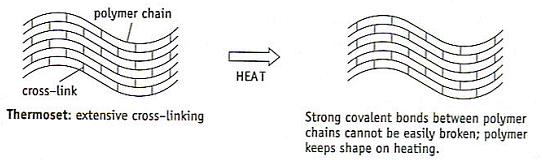
Thermosets
- These polymers have lots of corss-linking between the different polymer chains.
- These cross-links make the chains much stronger than in thermoplastics.
- The attractive forces cannot be broken by warming.
- The chains cannot move relative to each other and the polymer cannot change shape.
- If heated, the polymer just chars and burns. Bakelite is an example of a thermoset.
Baekeland
- The search for polymers originally concentrated on finding new substances to replace natural substances such as wood, rubber, cotton, silk and resins.
- Leo Baekeland set out to search for a replacement for shellac (used in varnishes); however he eventually discovered a resin, which when heated in a mould could be converted into a hard insoluble object with good insulating properties. He had produced the first synthetic polymer- bakelite.
- Baekeland and other chemists at the time had little understanding of the chemistry that was happening to make polymers.
- In the 1930’s, X-ray diffraction studies of solids showed that polymers contained large macromolecules.
- It was Herman Staudinger that proved that polymers contained large macromolecules.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home
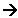 -- A-A-A-A –
-- A-A-A-A –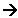 -- A-B-A-B –
-- A-B-A-B –
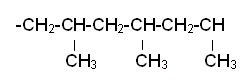


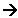 -- A-A-A-A –
-- A-A-A-A –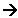 -- A-B-A-B –
-- A-B-A-B –