
Electrophillic Addition
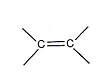
- The double bond in ethene give the region between the two carbon atoms a greater than usual negative charge.
- Cations (atoms with a partial fully positive charge) will be attracted to this negatively charged region.
- They can react with ethene, accepting a pair of electrons from the double bond.
- When species do this, they can be described as electrophiles.
- Bromine can be used to detect a C=C bond:
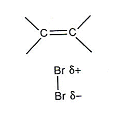
- When the Bromine molecule comes close to ethene, it becomes polarised by the negative charge of the double bond. That is, the electrons in the diatomic bromine molecule are repelled by the alkene and are pushed back along the molecule.
- The positively charged bromine atom acts as an electrophile, reacting with the double carbon bond.
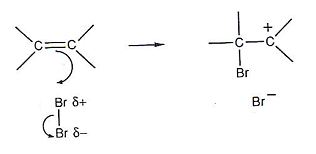
- A pair of electrons from the carbon double bond move onto the positive bromine atom.
- The electron pair between the two bromine atoms then move towards the other bromine atom, creating a bromine atom with a negative charge.
- One of the carbon atoms is now positively charged (it shares only six outer electrons).
- An ion like this is called a carbocation. Carbocations are very unstable.
- The carbocation goes on to react with the negative bromide ion.

- The negatively charged bromide ion is acting as a nucleophile.
- This detailed description of the reaction is known as a reaction mechanism.
- The overall reaction is:
C2H4(g) + Br2(g)  C2H4Br2(g)
C2H4Br2(g)
- The reaction is an addition reaction; it is an electrophillic addition reaction since the initial attack is by an electrophile.
- In the last stage of the reaction, the nucleophile can also be water, as water have lone pairs of electrons.
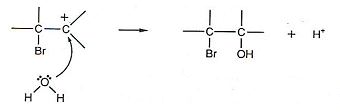
- In Bromine water, there may sometimes be more water molecules than bromide ions present, and so the above reaction would be more likely to occur.
- However, the bromine water would still be decolourised.
Reaction with Hydrogen Bromide
CH2=CH2 + HBr 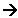 CH3CH2Br
CH3CH2Br
Reaction of ethene with water
- WITH A CATALYST OF PHOSPHORIC ACID, A TEMPERATURE OF 300oC, AND A PRESSURE OF 60atm
ethene can react with water: 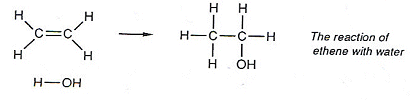 This reaction is important for the industrial manufacture of ethanol.
This reaction is important for the industrial manufacture of ethanol.
Reaction of ethene with hydrogen
- Also an addition reaction.
- The mechanism involves hydrogen molecules, and takes place on the surface of a catalyst (needed to help break the strong H-H bond).
- A platinum catalyst allows the reaction to take place under laboratory conditions; however a cheaper alternative of Nickel is used.
- Nickel is less efficient; the reaction occurs at 150oC and 5 atm of pressure (hydrogenation occurs).
CH2=CH2 + H2 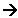 CH3-CH3
CH3-CH3
- Margarine is manufactured in this way. Plant and animal oils are “hardened” by hydrogenation.
- Unsaturated alkenes have a double bond; their bonding capacity is not used filled up.
- Polyunsaturates have more than one double C=C bond (margarine can be high in polyunsaturates).
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home




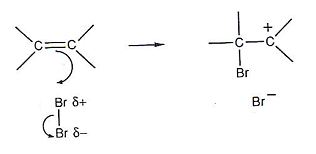

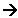 C2H4Br2(g)
C2H4Br2(g) 
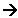 CH3CH2Br
CH3CH2Br

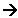 CH3-CH3
CH3-CH3