Alkenes
-
Alkenes are very much like alkanes in that they are hydrocarbons.
- Hydrocarbons contain carbon and hydrogen only.
- Alkenes differ from alkanes in that they have a C=C double bond.
- The presence of this C=C double bond makes the molecules unsaturated.
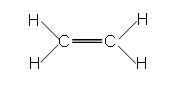
- Ethene is the simplest of all of the alkenes:

- The alkenes are named in the same way as the alkanes. Take pent-1-ene, the pent tells us that the longest chain contains five carbons, the ene suffix tells us its part of the alkene homologous series, and the number tells us where the C=C double bond is found.
- All alkenes have the same empirical formula:
CH2
- This is the same as the empirical formula for the cycloalkanes; however the double bond makes alkenes react differently to cycloalkanes.
- There are also cycloalkenes (for example cyclopentene), and there are also dienes, like penta-1,3-diene
Shapes of Alkenes
- Ethene is a planar molecule (all of the atoms are within the same plane.
- Ethene is a flat molecule, due to it being planar.
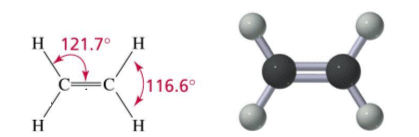
- The bonds around the C=C group are arranged the same in every alkene.
- This can be explained by saying that all of the electrons want to be as far away from each other as possible.
- The repulsion of the electrons is minimised when the bond angle is equal to 120o
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home