
Additional Polymers
- Under the right conditions, Alkenes can undergo polymerisation.
- The small unsaturated starting molecules are referred to as monomers, and they join together to form a long chain saturated polymer.
CH2=CH2 + CH2=CH2 + CH2=CH2  -CH2-CH2-CH2-CH2-CH2-CH2-
-CH2-CH2-CH2-CH2-CH2-CH2-
- The reaction requires a catalyst; originally the catalyst was O2, but an organic peroxide can be used.
- The mechanism involves radicals; the catalyst provides the radicals to get the reaction started.
- As the reaction involves radicals, it is made up of three stages; initiation, propagation, termination.
Initiation
- This involves creating a radical using the catalyst.
- If the catalyst is an organic peroxide, the weak O-O bond must first be broken to form a radical:
R-O-O-R  R-O. + R-O.
R-O. + R-O.
(R represents an alkyl group)
Propagation
- The radical now goes on to react with the alkene.
The radical combines with one of the double bonds in the alkene.
- The new radical goes on to react with more alkenes:
R-O-CH2-CH2. + CH2=CH2 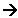 R-O-CH2-CH2- CH2-CH2.
R-O-CH2-CH2- CH2-CH2.
- The reaction is repeated many times, and so the chain grows longer and longer.
- The reaction is very rapid, chains of up to ten thousand monomers can be formed in seconds.
- As the chains grow, they flail around; sometimes “backbiting” can occur.
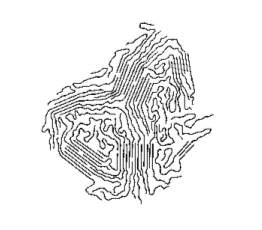
- The result of “backbiting” is to create a new growing point in the middle part of the chain. A branched chain will now grow; this is what occurs in the process used to make low-density polythene: there is around a branch per every 50 atoms (average).
Termination
- The reaction ends when all of the radicals are used up, one way this can happen is by radicals simply joining together.
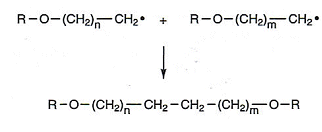
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home
 -CH2-CH2-CH2-CH2-CH2-CH2-
-CH2-CH2-CH2-CH2-CH2-CH2-
 -CH2-CH2-CH2-CH2-CH2-CH2-
-CH2-CH2-CH2-CH2-CH2-CH2-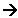 R-O. + R-O.
R-O. + R-O. 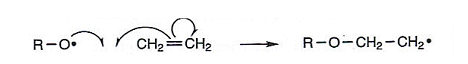
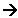 R-O-CH2-CH2- CH2-CH2.
R-O-CH2-CH2- CH2-CH2.

