
Ziegler Natta Catalysts
Ziegler Catalysts
- Ziegler was researching organometallic compounds (molecules that contain metals that are covalently bonded to carbon atoms).
- When testing an aluminium organometallic compound, strange things happened.
- He traced the unexpected behaviour down to tiny traces of nickel that had been left over in the apparatus.
- Ziegler and his team decided to focus their research on organometallic compounds mixed with transition metals.
- In 1953, they finally had success when they discovered that adding titanium compounds resulted in the formation of very long polymer chains.
- Simply passing ethene (atmospheric pressure, liquid form) into a solution containing a tiny amount of TiCl4 and (C2H5)3Al resulted in the immediate production of polyethene.
- The polyethene produced had a relative molecular mass of up to 3,000,000 with no branching along the polymer chain.
- This meant that the chains could be packed more closely than those made by the high pressure process.
- In this form the polymer is said to be crystalline (giving it higher density and strength).
- Hdpe (high density polyethene) is formed in this way.
- Hdpe is strong and can easily be moulded into complex shapes, and is able to retain its shape during heating. This means that it can be steam sterilized and is useful in hospitals for making equipment such as buckets and bed pans.
Natta- Stereoregular isomerism
- Stereoregular polymers are those with a regular structure.
- Natta was convinced that alkylaluminium catalysts were the key to making stereoregular catalysts.
- In 1954, he used Zieglerís catalyst to polymerise propene. He ended up with two forms of polypropene; a crystalline and amorphous (non crystalline) polypropene.
- He managed to separate the two different polymers.
- In the crystalline form the methyl groups all have the same orientation of the polymer chain (Natta named this form istotactic- meaning the same).
- The amorphous polymer had the methyl groups randomly orientated on the chain. He named this form atactic.
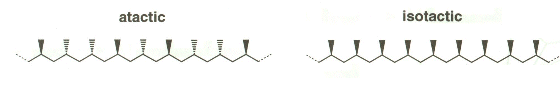
- Isotactic polymers:
- Have a regular structure and so make a crystalline tough structure (like hdpe).
- Are used in sheet and film form for packaging and containers; used to make fibres for carpets.
- Atactic polymers:
-
- Have an Irregular structure; chains are loosely held; itís soft and rubbery- like polyfiller.
- Are used to make roofing materials, sealants and other weatherproof coatings.
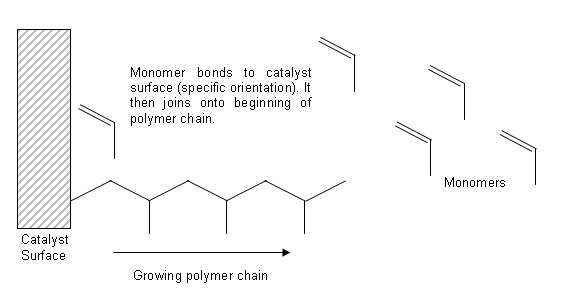
- Natta went on to develop new catalysts which allowed the polymer chains to grow out from the catalyst surface (like growing hair).
- These catalysts (Ziegler-Natta catalysts) have allowed chemists to tailor make specialist polymers with precise properties.
Metallocene catalysts
- After many years of research in the laboratory, a new kind of catalyst has been introduced into the polymer industry.
- The new catalysts are metallocenes.
- Metallocenes have a sandwich structure; a transitional metal atom is sandwiched in between two arene ring systems.
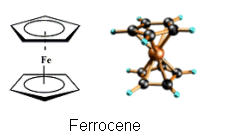
- Metallocenes are even more specific than Ziegler-Natta catalysts and give chemists even more control over the polymer chains that are produced.
- Polyethene and polypropene made with these catalysts have different properties to the normal plastics; for example they can be made into thin films, can be made stronger, impermeable and even tear resistant.
- These films are now been used in food packaging.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home



