
Crystalline Polymers
- Polymer chemists use the term crystalline to describe the areas in a polymer where the chains are packed in a regular way.
- Many polymers have a mixture of crystalline (ordered) areas and amorphous (unordered) regions.
- Amorphous regions have more freedom of movement as the chains are further apart.
- Any one polymer chain may be part of an amorphous region and a crystalline region.
- Spaghetti is a good representation of the different areas; when looking at the underside of a clear glass bowl containing spaghetti some regions of the spaghetti are grouped together in a regular layout, while other regions are totally mixed up.
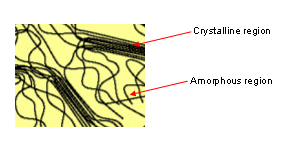
- Polymers with regular chain structures (e.g. isotactic polypropene) and without bulky side groups or extensive chain branching are the most likely to form crystalline regions.
- The percentage of crystallinity in a polymer is important in determining its properties; the more crystalline a polymer is, the stronger and less flexible it becomes.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home



