
Conducting Polymers
- The covalent molecular structures of polymers make them excellent electrical insulators, which is why they are often used to protect people from electricity.
- We are now able to make polymers that can conduct electricity; however their uses have not been exploited yet. Conducting polymers will probably play a great role in the future of plastics.
How do they work?
- When ethyne molecules polymerise, the product formed is polyethyne.
- Polyethyne molecules contain alternating single and double bonds.
- There are two geometric isomers of polyethyne as the double bonds can be arranged in different orientations to each other.
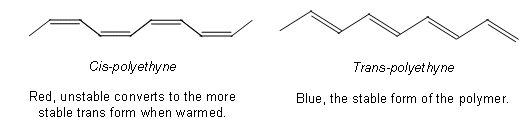
- Like many of the other polymers, the conducting polymer was made accidentally, when a student of Shirakawa (who first discovered the red polyethyne after directing a steam of ethyne onto a surface of a Ziegler-Natta catalyst at a temperature of -78oC) used 1000 times too much catalyst.
- One year later, the polymer’s use was found when it was “doped” with iodine, producing a metallic golden sheet that conducted electricity one million times better than previous forms of polyethyne.
- The race is now on to develop these polymers so that they become even better polymers and to find commercial uses for them.
Alkynes
 Alkynes are like alkenes in that they form another class of the unsaturated hydrocarbon family.
Alkynes are like alkenes in that they form another class of the unsaturated hydrocarbon family.
- However, instead of the C=C double bond that characterise alkenes, the alkynes have a carbon carbon triple bond.
- Alkynes are named in a similar way to the alkenes, but using an “-yne” suffix rather than “-ene”.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home



 Alkynes are like alkenes in that they form another class of the unsaturated hydrocarbon family.
Alkynes are like alkenes in that they form another class of the unsaturated hydrocarbon family.