Redox Reactions
- Oxidation can be described as a reaction in which hydrogen is lost or oxygen is gained.
- Reduction can be defined as a reaction in which hydrogen is gained or oxygen is lost.
For example,
CuO(s) + H2(g)  Cu(s) + H2O(l)
Cu(s) + H2O(l)
- In the above reaction copper oxide loses oxygen, so it has been reduced. Hydrogen gains oxygen so it has been oxidised.
- Oxidation and Reduction always occur together in what is called a redox reaction.
Redox in terms of Electron Transfer
- Redox reactions can also be described in terms of electron transfers.
- Reduction is the gain of electrons, and oxidation is the loss of electrons.

Consider the following reaction:
Mg(s) + Cl2(g)  MgCl2(s)
MgCl2(s)
- The above reaction shows the reaction between Magnesium and Chlorine.
- There are two processes occurring in the reaction above:
- Oxidation reaction: The magnesium is losing electrons:
Mg  Mg2+ + 2e-
Mg2+ + 2e-
- Reduction reaction: The chlorine is gaining electrons:
Cl2 + 2e- 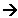 2Cl-
2Cl-
- These two equations are called half equations.
- The reducing agent is an electron donor, in the previous reaction it is the Magnesium.
- The oxidising agent is an electron acceptor (thief), in the previous reaction it is the Chlorine.
Oxidation Numbers
- Oxidation numbers are assigned to an atom or an ion to describe its relative state of oxidation or reduction. Using the oxidation numbers, it is possible to deduce whether a redox reaction has occurred and to identify the oxidising agent and reducing agent.
- Rules for assigning Oxidation numbers:

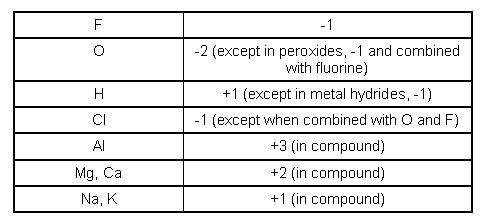
- If the oxidation number increases, the element is oxidised.
- If the oxidation number decreases, the element is reduced.
- The total change in an oxidation number increasing and decreasing in a reaction, must be the same in both directions.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home
 Cu(s) + H2O(l)
Cu(s) + H2O(l)
 Cu(s) + H2O(l)
Cu(s) + H2O(l)
 MgCl2(s)
MgCl2(s)  Mg2+ + 2e-
Mg2+ + 2e-