
Molecules and networks
- CO2 and SiO2 are both in group four of the periodic table, and so one might expect their physical properties to be similar; however CO2 is a gas at room temperature, whereas SiO2 is solid at room temperature and has an extremely high melting point.
- The difference between the substances is due to the dissimilarity between the bonds between carbon and oxygen, and silicon and oxygen.
- Both are covalent compounds, but because carbon is such a small atom it can form four bonds around it, making it capable of forming double bonds with oxygen; carbon dioxide is composed of individual molecules.
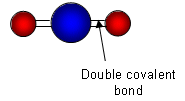
- The force between the individual carbon dioxide molecules is very weak, and so it doesn’t require much energy to separate them.
- Silicon atoms are larger than carbon atoms; they are able to bond to four oxygen atoms.
- Quartz (SiO2) is an extended network of SiO4 molecules, in which a central silicon atom is bonded to four oxygen atoms; each silicon atom has a half share of four oxygen atoms.
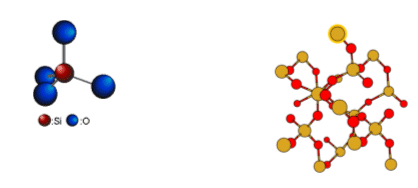
- Because of its “giant structure”, silicon dioxide is insoluble in water, and has a high melting and boiling point. The reason for this is the strong covalent bonds between the atoms, which require a lot of energy to break.
- There are two different types of covalent structure, depending on how the atoms bond with each other.
- Covalent molecular structures consist of molecules on their own. They have low melting and boiling points. The covalent bonds between the atoms within the molecules are strong, but the intermolecular forces are weak, and so don’t require a lot of energy to be broken.
- Covalent network structures are made from a network of repeating lattices of covalently bonded atoms. They often have high melting and boiling points and are insoluble in water.
Diamond and Graphite
- Diamond and graphite are both giant lattices made from carbon, and yet one of them is one of the hardest natural substances, and the other is used in pencils.
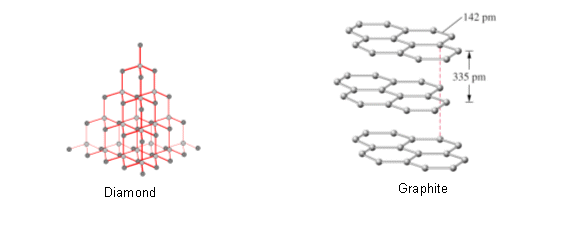
- The reason for their differences is their structures.
In diamond, each carbon atom is joined to four other carbon atoms tetrahedrally by strong covalent bonds. The structure is highly symmetrical and the C-C bonds are very strong, which is why it is the strongest naturally occurring substance.
- The structure of graphite is different to the structure of diamond. Rather than a carbon atom bonding to four other carbon atoms, each atom in graphite is bonded to three other atoms.
- This happens in each layer, forming a strong two dimensional network.
- The fourth outer shell electron contributes to the formation of a delocalised electron cloud between the layers. These delocalised electrons hold the layers together, but not very firmly.
- The delocalised electrons are also the reason it conducts electricity.
Fullerenes
-
In 1885, a new form of carbon was discovered; it occurred when a high power laser was used to evaporate graphite in an inert atmosphere.
- In 1890, lots more of the new carbon was produced when electricity was passed through graphite rods.
- The substance dissolved in benzene to give a red solution, which showed that it had a molecular rather than a network structure; however this puzzled scientists, as it had a formula of C60 which is very large.
- The new substance was named Buckminsterfullerene, because the structure was similar to a geodesic dome designed by Buchminster Fuller.
- The structure is similar to the structure of a football.
- Other carbon molecules with similar structures have been given the term fullerene.
- Recently elongated fullerenes have been discovered, these have been named “buckytubes” or “nanotubes” and if longer versions of these could be created, they could be used to create strong, lightweight structures.
- Fullerene’s properties have not yet been fully discovered, as they have only recently been discovered.
- Research is under way to find a possible use of fullerenes, and hundreds of patents have already been registered.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home






