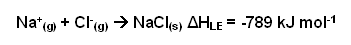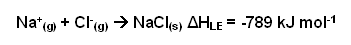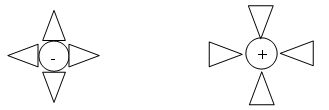Ions in solids and liquids
- Ionic substances are held together by their opposite electrical charges. Each cation is attracted to several anions and vice versa.
- The ions build up a giant lattice structure that is held together by strong ionic bonds (electrostatic charge).
- For an ionic substance to dissolve in a liquid, its ionic lattice must first be broken down into individual ions. The energy required to do this is known as the lattice enthalpy, for example:
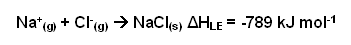
- All lattice energies are large negative values.
- As the ionic charge increases and the ionic radius decreases, the lattice enthalpy increases.
- When the ions dissolve in a solution they become highly disordered; they spread out throughout the liquid.
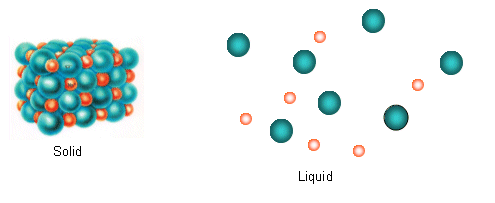
- The entropy increase is what causes substances to dissolve in solutions.
Ionic substances in a solution
- Lots of ionic substances dissolve readily in water. When this happens, the ions become surrounded by water molecules.
- When sodium chloride dissolves in water, the dissolved ions spread throughout the water randomly. The Na+ and the Cl- ions are separated and are totally independent of each other.
- This happens with all ionic substances when they dissolve; the positive and negative ions are separate and behave independently.
Ionic equations
- Ions in the solution behave independently, and so they must have separate chemical equations.
- The reactions of ionic substances often only include one of the ions, the other ion does not get involved in the reaction.
Example:
When a sodium chloride solution is mixed with a silver nitrate solution, a white precipitate is formed. This white precipitate is silver chloride. The silver cations and chlorine anions react to form a white precipitate; the sodium and nitrate ions are not involved in the reaction, they are described as spectator ions and are not involved in the chemical equation:
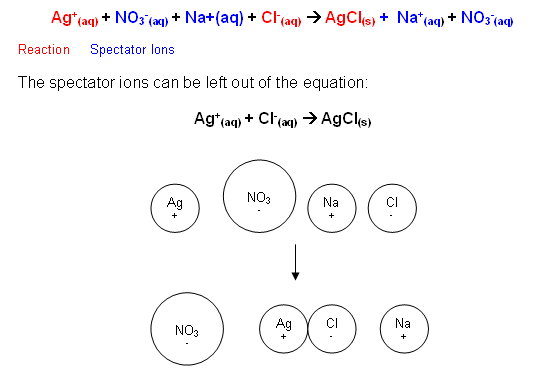
- An equation where the spectator ions have been left out is known as an ionic equation.
Hydration and Solvation
- Water contains polar molecules (positive and negative end):
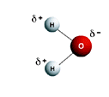
- The charges of the water molecule become attracted to the charges of the ions that dissolve in the water.
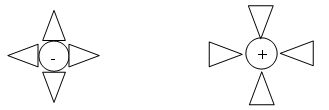
- The ions in the solution have water molecules attached to them; they are said to be hydrated.
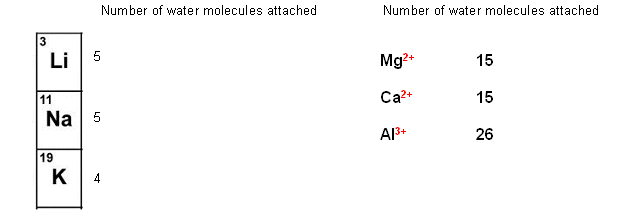
- The numbers of water molecules that can attach to an ion depend upon its charge, and its size; i.e. the smaller the ion and the stronger the charge, the more water molecules can be attached to it.
- When bonds form between the ions and the water molecules, energy is released. This energy could be high enough to pull more ions from the lattice.
- Water molecules are attracted to each other by hydrogen bonds, which need to be broken in order for the ions to bond to the water molecules. This process requires energy.
- It is a compromise between the energy needed to break the hydrogen bonds and ionic bonds, and the energy released when the ions become hydrated that determine whether a solid dissolves or not.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home