



 CuO(s) + SO2(g)
CuO(s) + SO2(g)The copper ore is crushed into 250mm pieces, and carried through a tunnel on a 5 mile conveyor belt. This is done so that the segments will be smaller, and therefore more able to float in the next process. At this point the ore is made up of approximately 0.5% of copper
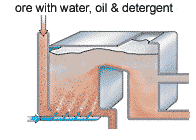
The purpose of the froth floatation is to separate the 0.5% of copper from the 99.5% of “crud”. There are two different types of minerals in the ore at this point; the chalcopyrite is a sulphide, and the rest are silicates containing anions made from silicon and oxygen atoms.
In this process, a “collector” is added to a water/mineral mixture. The collector binds to the surface of the chalcopyrite grains, giving them a water repellent nature. Detergent is added and air bubbles are blown through the mixture, carrying the water repellent carrier attached to the chalcopyrite. The chalcopyrite is now at the surface, ready to be removed.
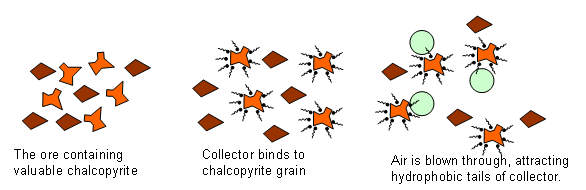
The air rises and the copper rises to the top, it can then be scooped off. The slurry of water is then disposed of in tailings pond. At this point the mixture contains 28% copper.
The smelter separates the copper from the sulphur in CuFeS2 in one stage:
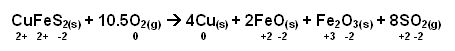
Copper and Oxygen are reduced, sulphur is oxidised, and iron is oxidised.
The iron oxides combine with silica which is added to the smelter, to form slag- a low density crust, which floats on the surface.
This stage is the final purification of copper; in this stage any copper sulphate is finally separated and the copper is removed. The copper solution is mixed with sulphuric acid. The cathodes are made from pure copper, and the anodes are made from impure copper.
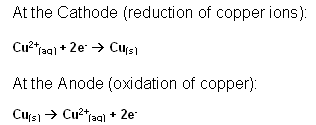
When the electricity is passed through the electrolyte, the copper ions on the anode dissolve into the solution, and are attracted to the cathode. At the cathode they pick up 2 electrons and become copper metal.
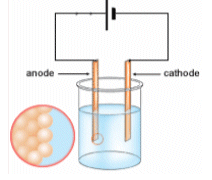
Some of the insoluble impurities in the solution are valuable (silver and gold), these are extracted from the sludge which forms. The copper is now 99.98% pure.