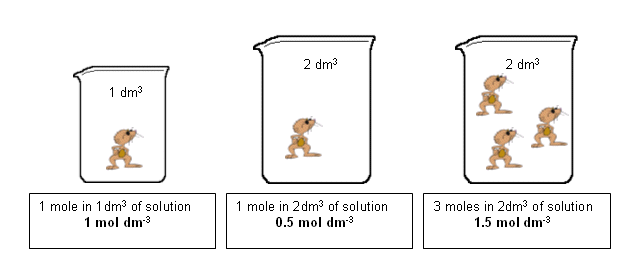


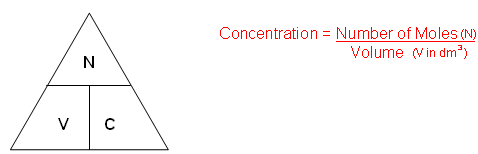
Example:
We have 250 cm3 of a solution of sodium hydroxide whose concentration is 2 mole dm-3. How many moles of sodium hydroxide do we have?
Rearrange the formula: Number of Moles (N)= Concentration (C) x Volume (V in dm3)
Change the units: 250 cm3 is the same as 0.25 dm3 (by dividing by 1000).
Substitute the values into the formula:
Number of Moles = 2 x 0.25
=0.5 moles
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)