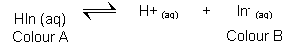Acid-Base Reactions
- An acid is a substance that turns litmus paper red, reacts with carbonates to give CO2 and is neutralized by bases.
- Acids have the ability to transfer H+ ions to something else.
- The substance which accepts the ions is called a base.
For example:
HCl (g) + NH3(g)  NH4+Cl- (s)
NH4+Cl- (s)
- In this reaction the HCl has transferred H+ ions and the NH3 has accepted the ions. The HCl is the acid, and the NH3 is the base.
- In the above reaction, the oxidation states of the elements remain unchanged.
- Since H+ ions only consists of only one proton, we can refer to acids as proton donors, and bases as proton acceptors.
- The theory of H+ transfer is known as the BrØnstead-Lowry theory of acids and bases.
Acids and Bases in Solutions
- Water consists mainly of H2O molecules, and hydrogen chloride is a gas that contains HCl molecules.
- When these two substances mix, the solution (Hydrogen Chloride in water) easily conducts electricity, so it must contain H+ ions.
- The reaction between the hydrogen chloride molecules and the water molecules produces these ions:
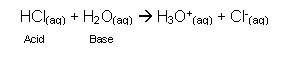
- In this reaction, the hydrochloric acid is the acid (it is the proton donor) and the water is the base (proton acceptor).
- The H3O+ ion is called the oxonium ion and it is present in every acidic solution. The acid donates H+ to H2O forming H3O+.
- The H3O+ can now act as an acid; it can donate an H+ ion to a base.
- The properties of an acid are the same as the properties of the H3O+ ion.
- The H3O+ ion is often shortened to just H+
HCl (aq)  H+ (aq) + Cl- (aq)
H+ (aq) + Cl- (aq)
- An acid dissolves in water to form H+ ions.
- An Alkali dissolves in water to form OH- ions.
- Some alkalis such as sodium hydroxide contain OH- ions, whereas others, such as ammonia, form OH- ions when they react with water.
NH3(aq) + H2O (l)  NH4+ (aq) + OH-
NH4+ (aq) + OH-
Acid-base pairs
- After an acid has donated its H+ ion, it can then accept it again:
CH3COOH (aq)  CH3COO- (aq) + H+ (aq)
CH3COO- (aq) + H+ (aq)
- When ethanoic acid acts as an acid, it donates its H+ ion (above).
- If a stronger acid is then added to the solution containing the ethanoic ions (CH3COO-), then they can act as a base, and accept H+ ions:
CH3COO- (aq) + H+(aq)  CH3COOH(aq)
CH3COOH(aq)
When the acid ion acts as a base, it is called a conjugate base. In the above reaction, the ethanoic ion is the conjugate base of ethanoic acid.
Every acid has a conjugate base, and every base has a conjugate acid.
We refer to them as conjugate acid-base pairs.
The general formula for a conjugate acid-base pair is summarised below.HA(aq)  H + A-
H + A-
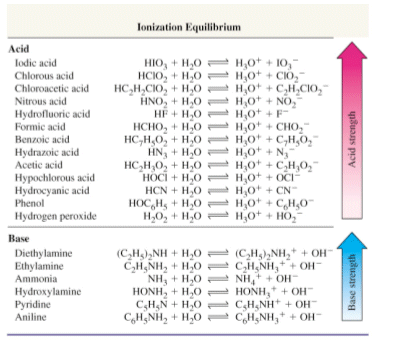
Strengths of acids and bases
- Acids can have different strengths, some are powerful H+ donors, whereas others aren’t quite as strong.
- Powerful H+ donors are called strong acids.
- Weak H+ donors are called weak acids.
- A strong acid has a weak conjugate base, and a strong base has a weak conjugate acid.
Indicators
- Most acid-base indicators are weak acids. The conjugate acid and base forms have different colours.
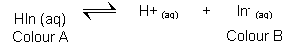
- When acid (H3O+) is added the position of equilibrium shifts to the left, producing colour A.
- When Alkali (OH-) is added the psoition of equilibrium shifts to the right, producing colour B.
- Litmus is an example of this, showing red in its base state when an acid is present, and blue in its acid state, when a base is added.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home
 NH4+Cl- (s)
NH4+Cl- (s)

 NH4+Cl- (s)
NH4+Cl- (s)
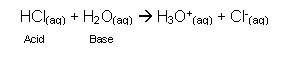
 H+ (aq) + Cl- (aq)
H+ (aq) + Cl- (aq)
 NH4+ (aq) + OH-
NH4+ (aq) + OH-
 CH3COO- (aq) + H+ (aq)
CH3COO- (aq) + H+ (aq)  CH3COOH(aq)
CH3COOH(aq)  H + A-
H + A-