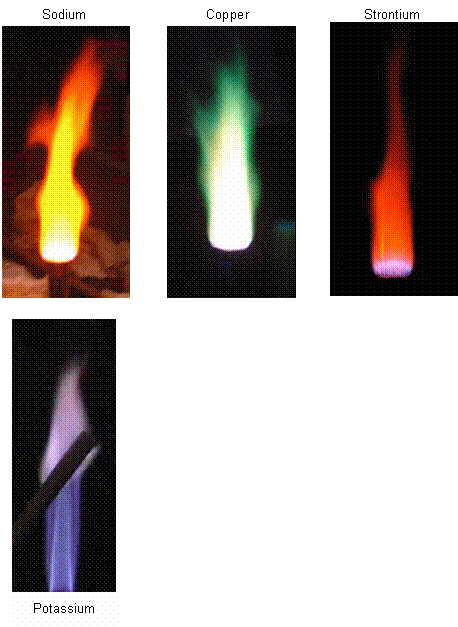Spectroscopy
- When atoms are given energy, their electrons jump up levels. The energy that is needed to cause the electrons to jump energy levels is specific.
- The atoms are “excited”, meaning they gain energy.
- After the atom electrons have been promoted, they get demoted again, that is they move back down the energy levels. When being demoted, the atoms emit the specific amounts of energy.
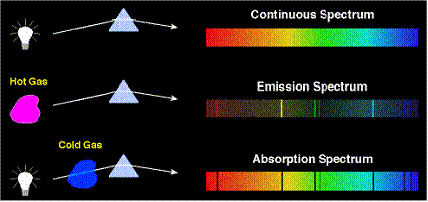
- This energy is in the form of light. When the light is viewed through a spectroscope, the light emitted is split up into an emission spectrum. The spectrum consists of a series of lines; the colour of these lines is specific to the wavelength.
- The frequency is related to the energy:
E=hv
- So, we can determine the energy that the electron emits during the demotion.
- Elements can be identified by their light emissions.
An absorption spectrum is when the light absorbed is analysed, and an emission spectrum is when the light produced is analysed.

Bohr’s theory of the Hydrogen Atom
-
Bohr’s theory uses the idea of quantisation of energy. The main points of Bohr’s theory were:
- The electron in the H atom is only allowed to exist in certain definitive energy levels.
- A photon of light is emitted or absorbed when an electron changes from one energy level to another.
- The energy of the photon is equal to the difference between the two energy levels.
- The frequency of the emitted or absorbed light is related to the energy energy by: E=hv.
- The uv emission spectrum of hydrogen can be related to the Lyman series. As the separate electrons demote, the energies are emitted. The spectrum lines become closer together the further from the nucleus. This is because the energy levels are closer together further from the n energy levels they are.
- When different elements are placed in a Bunsen burner flame, the flame becomes a different colour due to the specific energy level transitions. This is the same principle in the neon lights; the electricity causes the elements to give out certain light colours.
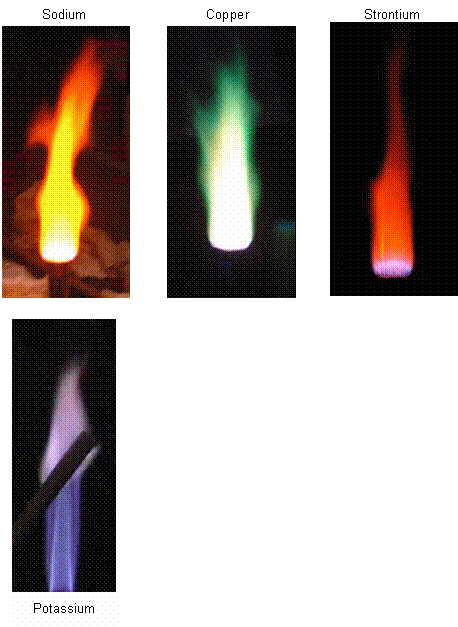
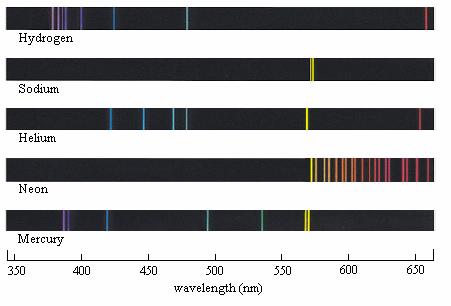
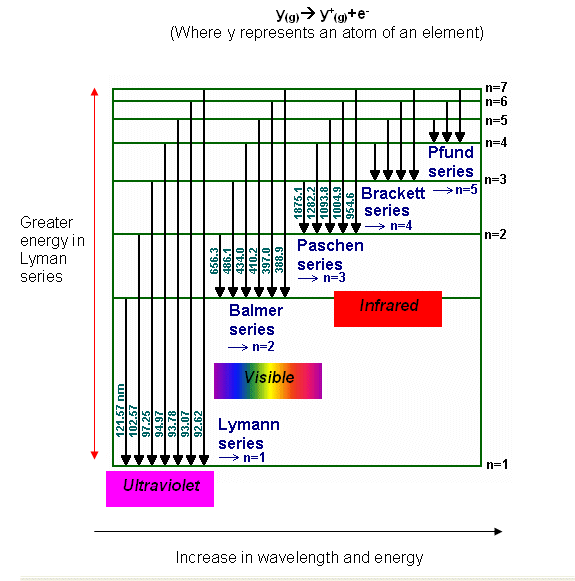
The Different series of Spectrograms
- Electrons that have been demoted from a high energy level to n=1 will emit more energy than one that has been demoted to n=2.
- The light energy emitted by electrons moving to the n=1 is within the ultraviolet part of the spectrum, as it contains more energy, The ultraviolet emission is studied using the Lyman series.
- The electrons being demoted to n=2 produce visible light, that is studied using the Balmer series.
- The energy levels after this are within the infrared spectrum and are studied using the Paschen and Bracket series.
- Where all the lines on the spectrograph become one is where ionisation has taken place, and the electron is no longer restricted to specific energy values. The frequency at where the lines converged can be used to determine the ionisation energy, using E=hv.
- The equation for an ionisation reaction is:
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home