|
The atomic size decreases as you go across the periods. This is due to the increase in the number of protons; as the number of protons increases, the pull on the energy levels increase, so the atomic size decreases. |
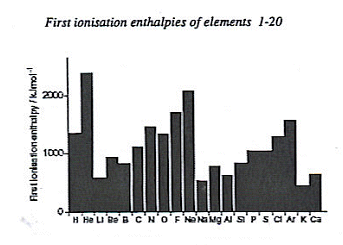 | The first ionisation of the elements increases as you go along a period, as there are more protons acting on the energy level. It then decreases as you move onto the next period, due to the extra energy level. |
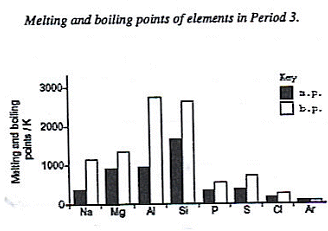 | The melting and boiling points increase along the period in the transition metals (due to their metallic structure) and then decrease in the gases. |




