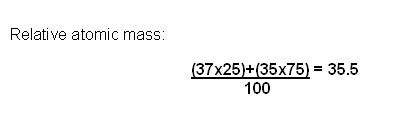Mass Spectrometer
The abundance of isotopes are determined using a Mass Spectrometer.
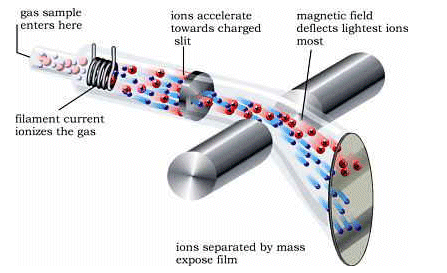
The Process
- Atoms vaporised, and sent into spectrometer.
- Heated tungsten wire produces electrons, which “knock electrons” from the sample, providing ions with a positive charge.
- Particles are accelerated using an electric field.
- A Magnetic field is placed 90o to the tube, the particles move;
they are deflected.
- The larger particles are deflected least, and the lighter particles most.
- The number of ions hitting the detector is measured, and the magnetic field
is changed so that the different isotopes can hit the detector. This gives
an abundance of each separate isotope.
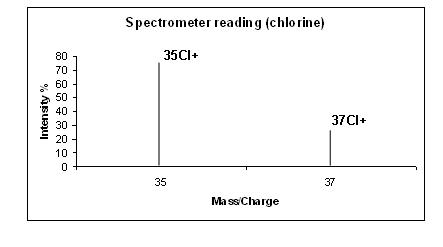
The results