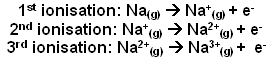Ionisation Energy
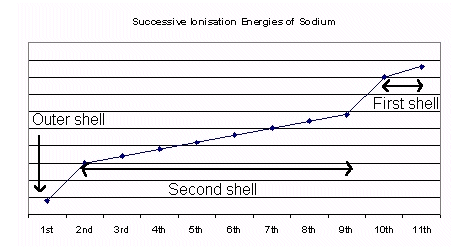
- The above graph shows the ionisation energies of sodium. Notice that where the jumps in the graph occur is where electrons are being taken from the next energy level.
- The equations for the ionisation of the electrons for sodium are:
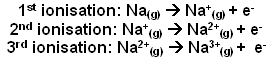
- After each successful ionisation, the following one becomes harder, this is because there is less shielding of the protons positive charge, and when electrons are being taken from the second shell, they are closer to the proton charge.
- The general equation for ionisation energies is:

- After each ionisation, a 1+ charge is added to the ion, and there is an extra electron added to the equation.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home