
Below is a graph showing the energy needed to “knock off” (ionise) each electron in potassiuml:
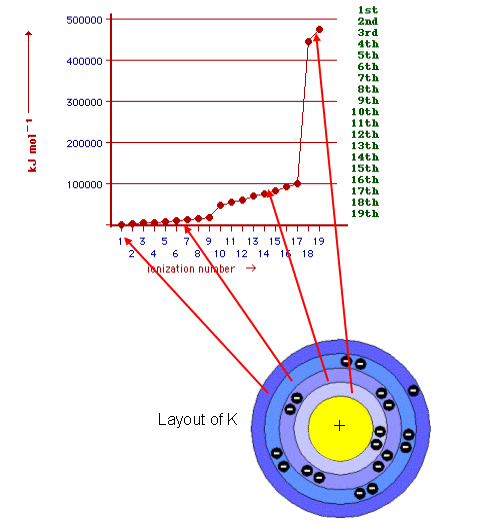
Notice that as the electrons are ionised from the outside inwards, the energy required increases, this is due to the electrons being closer to the nucleus (positive charge) for each successive energy level. Every time an electron is pulled off, the charge on all the other electrons is not shielded as much.