
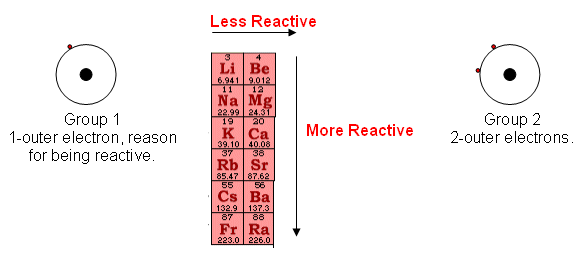
Groups one and two- The Alkali Metals
- Weak bonds between atoms (reason for it being soft and easy to cut.
- Never found native in nature.
- Much of the ground beneath us is made out of elements from the s-block, for example magnesium and calcium.
- There are similarities between the groups because of their electron configuration, but differences are because of their different masses.
- React vigorously with water to form a metal hydroxide and hydrogen.
Metal + Water 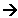 Metal Hydroxide + Hydrogen
Metal Hydroxide + Hydrogen
M(s) + H2O 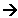 M(OH)2 + H2
M(OH)2 + H2
Remember Squeeky pop!!
- React with oxygen to form Metal Oxide.
- The strongest hydroxides and oxides are those closest to the bottom of the group.
- The elements towards the bottom of the group are more reactive, because the one outer electron is further away from the positive nucleus, so the force needed to take the electron away is comparatively less than the elements toward the top of the group.
- The hydroxides and oxides form alkaline solutions in water.
- The hydroxides and oxides can be neutralized with acid to form salts.
MO(s) + 2HCl(aq) 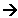 MCl2(s) + H2O(l)
MCl2(s) + H2O(l)
M(OH)2(s) + H2SO4(aq)  MSO4(s) + 2H2O(l)
MSO4(s) + 2H2O(l)
- The neutralizing effect used to neutralize field acidity.
- The general formula of group 2 carbonates is
MCO3 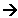 MO(s) + CO2
MO(s) + CO2
- When you heat the carbonates they decompose to form the metal oxide, and carbon dioxide.
- The carbonates become harder to decompose as you go down the group. The thermal stability of the elements increases as you go down the groups.
- The solubility of the elements in hydroxides (OH-) increases as you go down the group. This is common where the negative ion has a single charge (1-)
- The solubility of the elements in carbonates (CO32-) decreases as you go down the group. This is common where the negative ion has a double charge (2-).
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home



 Metal Hydroxide + Hydrogen
Metal Hydroxide + Hydrogen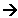 M(OH)2 + H2
M(OH)2 + H2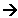 MCl2(s) + H2O(l)
MCl2(s) + H2O(l)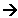 MSO4(s) + 2H2O(l)
MSO4(s) + 2H2O(l)
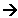 MO(s) + CO2
MO(s) + CO2