
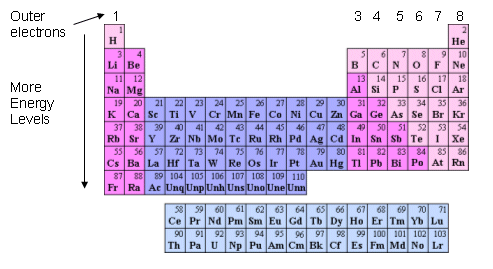
Relationships between the position of an element in the periodic table and its electron structure
- The group in which the element is found is how many electrons are in the outer energy level. For example group one elements have one outer electron, and group seven elements have seven outer electrons.
- As you move along the period, the atomic number increases, therefore the number of protons and electrons increases. This means that as you move from left to right along a period an electron is added to the outer energy level.
- All elements within the same period have the same number of energy levels.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home


