Chemical
Formulae
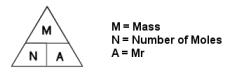
To
work out the number of moles in an element/compound, just divide the mass by
the Ar or the Mr.
For
example:
“How many moles are there in 32g of oxygen?”
Ar of oxygen is 16
So 32/16 = number of moles
Number of moles = 2
To
work out the mass, multiply the number of moles by the Ar
or Mr.
For
example:
“What is the mass of 6 moles of Carbon?”
Ar of Carbon is 12
So 12 x 6 = Mass
Mass= 72g
To work out the number of particles, just multiply the number of moles by
Avagadro's constant
“How many particles are there in 2 moles of carbon?”
2 x 6.02 x1023
= 1.2 x 1024
To work out the empirical formulae of a substance,
the mass is needed.
For
example:
“0.020g of Hydrogen combine with 0.168g
of Oxygen. What is the empirical formula of Water?”
Mass: 0.020g H 0.168g O
Number of Moles: 0.20/1 0.168/16
Ratio: 0.02 :
0.01 = 2:1 so the formula is H2O
To
work out the molecular formula, divide the Molecular Mass by the sum of the
Atomic masses of the empirical formula.
For
example:
“The empirical formula of methane is CH3,
the formula mass of methane is 30, work out the molecular formula”
CH3 = 12 + (3x1)
= 15
30/15= 2
so the molecular formula is twice the empirical formula.
C2H6
