Balancing
equations- Key Steps!
- List out the
different atoms in the formula.
- Count the number of
atoms on the left hand side, then on the right hand side.
- Note which atoms
are not balanced.
- Select on atom to
balance (it is usually the one that is an odd number). You can only balance by putting numbers in front of the
elements!
- Update the atom
count.
- If it is still
unbalanced, you may need to try balancing the elements again.
Example
Al + O2 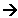 Al2O3
Al2O3
|
Atom |
Number of Atoms |
|
Al |
1 |
|
O |
2 |
|
Atom |
Number of Atoms |
|
Al |
2 |
|
O |
3 |
Oxygen is unbalanced!
Al + O2 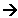 2Al2O3
2Al2O3
|
Atom |
Number of Atoms |
|
Al |
1 |
|
O |
2 |
|
Atom |
Number of Atoms |
|
Al |
4 |
|
O |
6 |
Now Aluminium and
Oxygen are unbalanced, but we can change the left hand side of the equation to
balance both sides.
4Al + 3O2 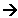 2Al2O3
2Al2O3
|
Atom |
Number of Atoms |
|
Al |
4 |
|
O |
6 |
|
Atom |
Number of Atoms |
|
Al |
4 |
|
O |
6 |
Now both sides are balanced!
