
Volumes of Gases
-
The number of molecules in one mole of gas is always 6 x 1023 (Avagadro's constant).
- The molecules of a gas are extremely spread out, and so the size of them is negligible compared to the total volume the gas occupies.
- So one mole of gas always occupies the same volume, and at standard conditions (1 atmosphere, 298K) that volume is 24dm3.
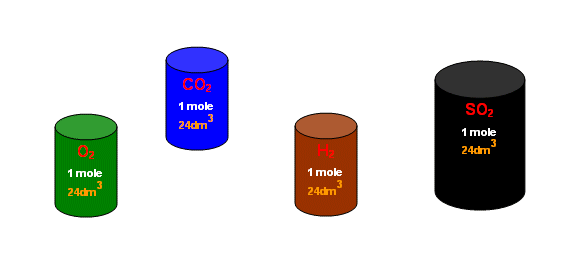
-
We can use this idea of molar volumes to work out the volumes of gases involved in a chemical reaction. For example,
Ammonia is produced from Hydrogen and Nitrogen:
N2(g) + 3H2(g) 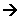 2NH3
2NH3
From the equation, 1 mole of Nitrogen reacts with 3 moles of Hydrogen to produce 2 moles of Ammonia.
So, using this idea:
(1 x 22.4dm3) N2(g) + (3 x 22.4dm3) H2(g) 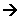 (2 x 22.4dm3) NH3(g)
(2 x 22.4dm3) NH3(g)
So if we had 10dm3 of Nitrogen, it would react with 30dm3 of Hydrogen to form 20dm3 of Ammonia (standard conditions).
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home



 2NH3
2NH3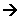 (2 x 22.4dm3) NH3(g)
(2 x 22.4dm3) NH3(g)