
Pollution
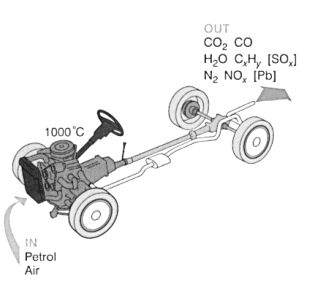
- Cars produce lots of emissions that are harmful to the environment. These emissions are causing great concern around the world.
- Cars emit carbon dioxide, carbon monoxide, water, un-burnt hydrocarbons, sulphur dioxide, nitrogen and nitrogen oxides.
- Hydrocarbon emissions result when the fuels in engines donít burn completely. They are also released during refuelling, and during hot days when the petrol evaporates. Hydrocarbons react in the presence of nitrogen oxides and sunlight to form ground level ozone (smog). Ozone irritates the eyes, damages the lungs and aggravates respiratory problems.
- Nitrogen Oxides are formed when nitrogen and oxygen atoms in air react under high temperatures and pressures present in the engine. Nitrogen oxides, like hydrocarbons, lead to the formation of ozone. They also lead to acid rain.
- Carbon monoxide is formed from the incomplete combustion of hydrocarbons, and occurs when carbons in the fuel are partially oxidised, rather than being fully oxidised. Carbon monoxide is a poisonous gas, as it bonds with haemoglobin in the blood.
- Carbon dioxide is produced when hydrocarbons are burned. Carbon dioxide is not poisonous, but it is a greenhouse gas and leads to excessive global warming.
- Sulphur Dioxide is formed from impurities in petrol. Sulphur dioxide leads to acid rain.
Catalysts
- A catalyst is a substance which alters the rate of a reaction without undergoing any permanent damage.
- Catalysts are not used up during a reaction (so they can be reused), and they are not damaged chemically during a reaction.
- However, catalysts can become physically damaged, for example the surface of the catalyst can crumble.
- Only small amounts of catalysts are needed in a reaction, as they are being re-used.
- Catalysts only affect the rate of the reaction, not the amount of product formed. Catalysts usually speed up chemical reactions; however some catalysts slow down reactions and these are called inhibitors, or negative catalysts.
- Catalysts are usually specific for one type of reaction, as are biological catalysts (enzymes).
- Catalysts can be added to cars (catalytic converters) in order to reduce the amount of pollution being emitted.
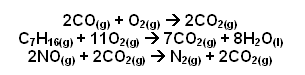
- In these reactions the pollutants are being converted to carbon dioxide, water and nitrogen which are naturally present in the air. These pollutions do react on their own, but catalysts make them go faster.
 Catalysts are present in catalytic converters.
Catalysts are present in catalytic converters.
- A lean burn engine uses an oxidation catalyst which removes CO and Hydrocarbons, converting them to carbon dioxide and water. This catalyst system does not remove nitrogen oxides.
- Catalytic converters can be fitted to ordinary cars; instead of an oxidation catalyst, a three-way catalyst system is needed, which catalyses oxides, CO, hydrocarbons, and nitrogen oxides.
- This catalyst only works if the air-petrol mixture is controlled so that it is exactly stoichiometric.
- The reduction catalyst is made from platinum and rhobium and it reduces NOx emissions.
2NO(g)  N2(g) + O2(g)
N2(g) + O2(g)
or
2NO2  N2(g) + 2O2(g)
N2(g) + 2O2(g)
- The oxidation catalyst is made from platinum and palladium and it is used to lower emissions of un-burnt hydrocarbons and carbon monoxide.
2CO (g) + O2(g)  2CO2(g) .
2CO2(g) .
Types of Catalysts
- If the reactants and catalysts are in the same physical state, for example they are both in an aqueous solution), then the reaction is said to involve homogenous catalysis.
- In many cases, the catalyst and reactants are in a different physical state. This kind of reaction is said to have involved heterogenous catalysis.
How Catalysts Work
- All chemical reactions require bond breaking, and bond making.
- Breaking the bonds requires energy known as the activation energy. The energy supplied must be higher than the activation energy, in order for the reaction to occur.
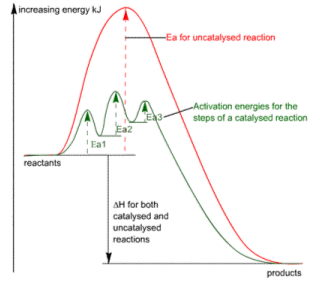
- If the activation energy is high, very few molecules will have enough energy to overcome it, and so the reaction will take place at a slow rate.
- Catalysts speed up chemical reactions by providing an alternative pathway for breaking and remaking bonds.
- In the reaction above, the activation energy is made lower, and so more bonds can be broken and reformed, hence a faster reaction.
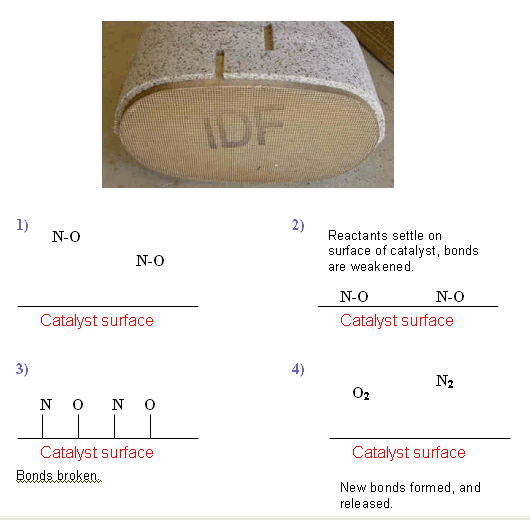
How a Heterogeneous catalyst works
- When a solid catalyst is used to speed up the reaction between liquids or gases, the reaction occurs on the surface of the catalyst.
- This is why it is important for the catalyst to have a high surface area, so that more of the reactants can react on the surface.
- Solid catalysts are usually found in a finely divided form or as a fine wire mesh. In catalytic converters the catalyst is supported on a porous material to increase its surface area.
Catalyst poisoning
- Catalyst poisoning occurs when other gases are absorbed more readily onto the catalyst than the reactants. This prevents the reactants from doing so. Catalyst poisons in cars include lead and sulphur.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home



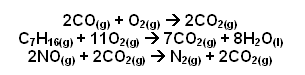
 Catalysts are present in catalytic converters.
Catalysts are present in catalytic converters.
 N2(g) + O2(g)
N2(g) + O2(g)  N2(g) + 2O2(g)
N2(g) + 2O2(g)  2CO2(g) .
2CO2(g) . 
