Hess's Energy Cycle
Enthalpy changes using combustion data
- Many enthalpy
changes of formation reactions do not occur under standard conditions, and
so they cannot be directly measured; however they can be measured indirectly
using energy cycles.
- Elements react
to form a compound. When the compound is combusted it forms the same substances
as when the elements are combusted. Using this relationship we can work
out the standard enthalpy of formation indirectly.
∆Hc1= ∆Hf + ∆Hc2
- The total
enthalpy of combustion for the reactants is equal to the formation
energy of the products plus the combustion
energy of the products.
- This makes
sense as Energy can neither be created nor destroyed, so as long
as the starting point and the finishing point are the same, then the enthalpy
change will always be the same no matter how you get from start to finish.
- So if a process
can be written in several steps, the enthalpy change of the whole process
is equal to the sum of the steps.
To find out the Enthalpy change using Heats of combustion:
- Start with an
equation to represent the energy change.
- Using the combustion
data complete a Hess law cycle.
- Balance the cycle
and add symbols representing ∆H1 ∆H2 and
∆H3.
- Remember to get
the direction of change correct (arrows go form reactants and products down
to the combustion products).
- Use Hess's law
to find the relationships between the enthalpy changes and rearrange to find
the value wanted.
Example:
Find the Enthalpy Formation Energy of Methane.
-
So, if
we can measure ∆Hc2 and ∆Hc1, we can work
out ∆Hf.
∆Hf =
∆Hc2 - ∆Hc1
-
It is minus ∆Hc1 because the reaction
is going in the opposite direction to what we want.
-
∆Hc2 is the
sum of enthalpy changes of combustion of 1 mole of carbon and 2 moles of hydrogen
and ∆Hc1 is the
enthalpy change of combustion for methane, and ∆Hf
is the enthalpy change of formation for methane, which is what we are
trying to find out.
∆Hf = ∆Hf (CH4)
∆Hc2 =
∆Hc(C) + 2∆Hc(H2)
∆Hc1 = ∆Hc(CH4)
So:
∆Hf(CH4) = {∆Hc(C) +
2∆Hc (H2)} - {∆Hc (CH4)}
= -393 + 2(-286)
- (-890)
=-75 kJ mol-1
Enthalpy changes
using formation data
- Sometimes it
is necessary to use enthalpy change of formation to work out the enthalpy
change of combustions.
∆Hf1 =
∆Hf2 + ∆Hc
- The Enthalpy
change from the elements in their standard states to the products
is equal to the formation energy of the products plus the Enthalpy change from the elements in their standard states
to the reactants.
To find out
the Enthalpy change using Heats of combustion:
1.
Start with an
equation to represent the energy change.
2.
Using the formation
data complete a Hess law cycle.
3.
Balance the cycle
and add symbols representing ∆H1 ∆H2 and
∆H3.
4.
Remember to get
the direction of change correct (arrows go form elements in standard states
to the reactants and products).
5.
Use Hess's law to
find the relationships between the enthalpy changes and rearrange to find the
value wanted.
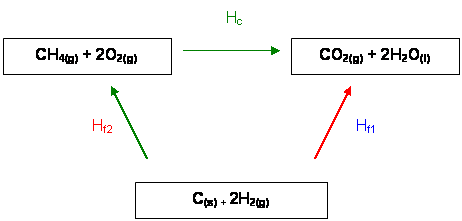
Example:
Find the enthalpy
change for the following reaction:
CH4(g) + 2O2(g) 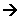 CO2(g)
+ 2H2O(l)
CO2(g)
+ 2H2O(l)
∆Hf1 =
∆Hf2 + ∆Hc
∆Hc = ∆Hc
(C) + ∆Hf (CO2(g))
+ 2∆Hf (H2O(l))
∆Hf2 =
∆Hf2(CH4(g))
∆Hf1 = ∆Hf2
+ ∆Hc
So ∆Hc
= ∆Hf1 -
∆Hf2
= ∆Hf (CO2(g)) + 2∆Hf
(H2O(l)) - ∆Hcf(CH4(g))
= -394 + - 572 - -75
= -891 KJ mol-1
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home

 CO2(g)
+ 2H2O(l)
CO2(g)
+ 2H2O(l)