
Organic Halogen Compounds
-
Organic Halogen compounds contain one or more halogen atoms (F, Cl, Br or I) attached to a hydrocarbon chain.
- Organic halogen compounds don’t occur naturally.
- The halogen atom modifies the un-reactive hydrocarbon chain, making it reactive.
- halogenoalkanes are the simplest form of organic halogen compounds (halogen atom attached to an alkane chain).
- The halogenoalkanes are named after their parent alkanes, using the same rules as learnt in Developing fuels:
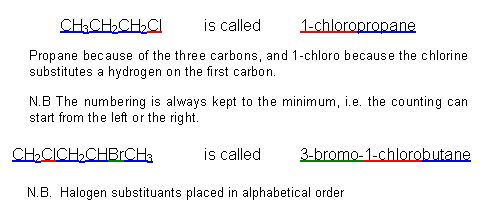
- The properties of the halogenoalkanes depend upon which halogen atom they contain.
Physical properties
- The carbon-halogen bond is polar, but it doesn’t make a big difference to the physical properties of the compounds.
All halogenoalkanes are immiscible with water, but dissolve in organic compounds.
- Their boiling points depend upon the size and the number of halogen atoms present: an increase in the size of the halogen atom and the more of them there are, the higher the boiling point.
- The influence of halogen atoms on the boiling point is important when it comes to designing compounds for particular purposes (i.e. refrigerants).
- If you want a halogenalkane with a high boiling point, you would add a larger halogen atom (i.e. one from lower down the group).
- However this causes a problem, as the heavier ones cause environmental damage.
- These factors have to be considered when looking for a replacement for CFC’s.
Reactions with halogenoalkanes
- Reactions of the halogenoalkanes involve the breaking of any carbon-halogen bond, which can break both homolytically or heterolytically.
- Homolytic fission forms free radicals. It occurs when the correct frequency of radiation is absorbed by the halogenalkane:
CH3Cl + hv  CH3. + C.
CH3. + C.
- This reaction occurs in the stratosphere where the halogenoalkanes are exposed to UV radiation.
- This reaction forms chlorine free radicals which catalyse the decomposition of the ozone layer.
- Heterolytic fission is common under ambient conditions in the troposphere; the reactions tend to be carried out in a solution.
- The carbon-halogen bond is already polar and it can break when enough energy is provided forming a negative halide ion and a carbocation (positively charged carbon atom):
CH3Cl  CH3+ + Cl-
CH3+ + Cl-
- These ions don’t last for long, as they react with the other substances in the solution.
Reactivity of the halogenoalkanes
- All reactions of halogenoalkanes involve the breaking of the carbon-halogen bond.
- The stronger the C-Hal bond is, the harder it is to break.
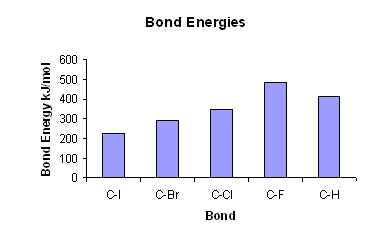
- The higher the bond energy, the stronger the bond. The bond energies are stronger higher up the group, as the halogens are smaller.
- The high bond energy of C-F bond makes it hard to break; this is why fluoro compounds are very un-reactive.
- As you move down the group seven elements, the C-Hal bond gets weaker, thus the compounds get more reactive.
- Chloro compounds are un-reactive and so stay in the environment for a long time.
- This means that substances such as CFC’s can last long enough to make it into the stratosphere, where they catalyse the destruction of the ozone layer.
- Bromo and Iodo compounds are fairly reactive, which makes them useful as intermediates in organic compound synthesis.
Substitution Reactions
- Substitution reactions are common with the halogenoalkanes.
- An example of a substitution reaction is when a halogenalkane and hydroxide ions are reacted together. The halogen alkane is hydrolysed to form an alcohol:
CH3-CH2-CH2-CH2-Br + OH-  CH3-CH2-CH2-CH2-OH + Br-
CH3-CH2-CH2-CH2-OH + Br-
- This substitution reaction occurs, because the C-Br bond is polar and the oxygen atom within the hydroxide group is negatively charged.
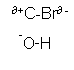
- The partial positive charge on the carbon atom attracts the negatively charged oxygen of the hydroxide ion.
A lone pair of electrons on the oxygen atom forms a bond with the C atom as the C-Br bond breaks.
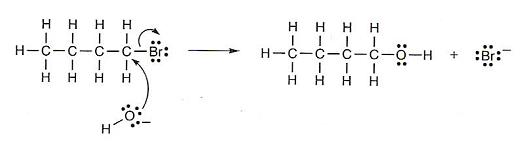
- The reaction involves heterolytic fission as ions have been formed rather than free radicals.
- A carbocation is not formed as the OH- ion reacts at the same time as the C-Br bond breaks.
- A full pair of electrons has moved in the reaction, rather than just one electron during homolytic fission.
- halogenoalkanes can react with many other reagents other than hydroxide ions. All that is needed is a group carrying a pair of electrons to start forming a bond to the carbon atom.
- These attacking groups which donate a pair of electrons to a positively charged carbon atom to form a covalent bond are called nucleophiles.
- These nucleophiles can either be negative ions like OH or molecules with a lone pair of electrons (H2O and NH3 etc.)
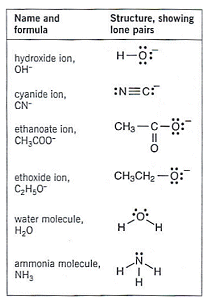 The table to the right shows some common nucleophiles.
The table to the right shows some common nucleophiles.
- The carbon atom that is attacked by the nucleophile can either be part of a carbocation (carrying a full positive charge) or it may be part of a neutral molecule (such as in the bromobutane reaction previously) carrying a partial charge due to bond polarisation.
- The general nucleophilic substitution can be described by the reaction below (X- represents the nucleophile):

- When a nucleophile reacts with a halogen alkane, the following reaction occurs:
R-Hal + X-  R-X + Hal-
R-X + Hal-
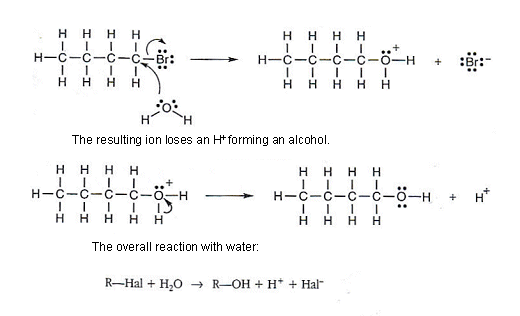
Water acting as a nucleophile
- A neutral molecule can act as a nucleophile in addition to just negatively charged molecules.
- Provided that the molecule has a lone pair of electrons it can act as a nucleophile.
- Water molecules have a lone pair of electrons on the oxygen atom (see table above).
- Water can act as a nucleophile, attacking a halogen alkane molecule.
- The reaction occurs in two stages:
Ammonia acting as a nucleophile
- As ammonia has a lone pair of electrons it can also act as a nucleophile:
- The lone pair of electrons on the N atom attacks the halogenalkane.
The product is an amine with an NH2 group.
R-Hal + NH3  R-NH3+ + Hal-
R-NH3+ + Hal-  R-NH2 + H+ + Hal-
R-NH2 + H+ + Hal-
Nucleophilic substitution to make halogenoalkanes
- When a halogenalkane reacts with OH- ions, a nucleophile reaction occurs and an alcohol is formed.
- The reverse of this reaction can be used to form a halogenalkane from an alcohol, providing the conditions are correct.
- It is another nucleophilic reaction; however it is the Hal- that is the nucleophile.
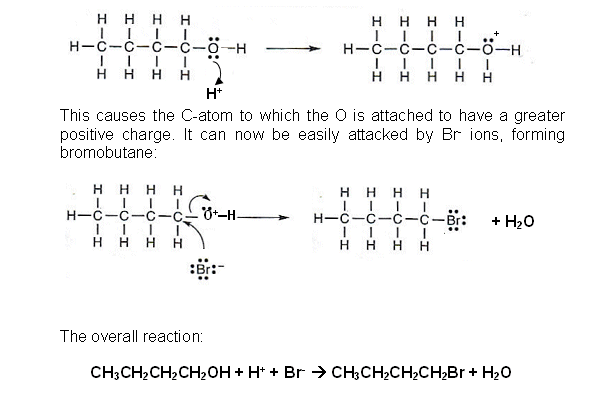
- The overall reaction:
CH3CH2CH2CH2OH + H+ + Br-  CH3CH2CH2CH2Br + H2O
CH3CH2CH2CH2Br + H2O
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home



 CH3. + C.
CH3. + C.  CH3+ + Cl-
CH3+ + Cl-
 CH3-CH2-CH2-CH2-OH + Br-
CH3-CH2-CH2-CH2-OH + Br- 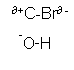

 The table to the right shows some common nucleophiles.
The table to the right shows some common nucleophiles.

 R-X + Hal-
R-X + Hal-
 R-NH3+ + Hal-
R-NH3+ + Hal-  R-NH2 + H+ + Hal-
R-NH2 + H+ + Hal-

 CH3CH2CH2CH2Br + H2O
CH3CH2CH2CH2Br + H2O