
Free Radicals
-
All reactions involve the breaking and reforming of bonds.
- Breaking bonds can be referred to as bond fission. The way bonds break can have an influence upon reactions.
- Covalent bonds involve the sharing of electrons:
H:Cl
- When the bonds break, the electrons are redistributed between the two atoms. There are two ways in which this can happen.
Homolytic Fission
- In this type of fission, one of the shared electrons goes to each atom:
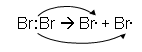
- The dot shows that each Br atom has gained an electron from the shared pair.
- The Atoms have no overall electric charge, because they have both regained their electron that they submitted to the shared pair.
- An unpaired electron has a tendency to pair up again with another electron from another substance.
- These highly reactive atoms (or groups of atoms) with unpaired electrons are called free radicals.
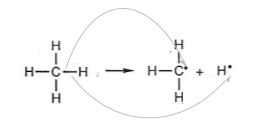
- Another example is when the C-H bond in methane is broken:
Heterolytic Fission
- In this type of fission, when the bond breaks, the electrons just go to one of the atoms.
- This atom becomes negatively charged, because it has an extra electron.
- The other atom becomes positively charged.
H:Cl  H+ + :Cl-
H+ + :Cl-
- When the bonds holding HCl together are broken, both of the electrons go to the chlorine atom, making it a negative ion. The Hydrogen atom is short of one electron, so it becomes a positive ion.
- Heterolytic fission is common in molecules with polar bonds.
- Bromomethane contains a C-Br bond, which can break heterolytically. The Bromine atom is more electronegative so it takes both of the electrons.
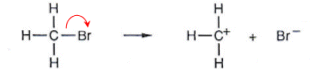
- The bromine atom becomes negatively charged; it becomes a nucleophile.
- The CH3 molecule becomes positively charged; it becomes an electrophile.
- Polarity occurs when there is an electronegativity difference between two bonded atoms; bromine is more electronegative than carbon; it has a greater electron pulling power.
- For many chemical reactions, the conditions in which they take place can determine how a bond breaks. For example, when the bromomethane is dissolved in a polar solvent, the C-Br bond breaks heterolytically; however in a non polar substance it breaks homolytically.
Free Radicals
- The key feature of a free radical is the unshared electron.
- The unshared electron makes it very reactive.
- Some free radicals are not as reactive as others, and can last long enough to behave as normal molecules. Nitrogen monoxide is an example.

- The nitrogen atom only has seven electrons in its outer energy level, the odd unpaired electron makes it a free radical.
- Di-oxygen is also a stable free radical, because of the two unpaired electrons.

Why are free radicals so reactive?
- Filled outer electron shells are more stable than unfilled ones.
- Free radicals are so reactive because they tend to grab electrons from other atoms or molecules to fill their outer energy levels.
- For example a chlorine free radical will grab an electron from a pair of hydrogen atoms, forming a new bond between a hydrogen and a chlorine atom and making a hydrogen free radical.

- The
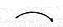 shows the movement of one electron.
shows the movement of one electron.
- The hydrogen free radical is also very reactive, so this again can create another free radical, the process continues.
- The reaction between the chlorine and hydrogen atoms is a typical photochemical free radical reaction; it is light that provides the energy for it to happen.
- Free radical chain reactions occur in three stages:
Stage one: Initiation
- A photodissociation reaction usually occurs to initiate the process, for example chlorine molecules are decomposed by light, forming two chlorine free radicals:
Cl2 + hv  2Cl.
2Cl.
- Only two chlorine free radicals are formed, but these initiate the process.
Stage two: Propagation
- The free radicals now react with other molecules, creating more free radicals.
- In our example, the chlorine free radicals react with hydrogen:
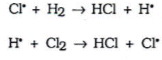
Stage three: Termination
- Eventually two free radicals will collide with each other (not very common).
- When this does happen, the radicals lose some of their energy when forming a new molecule. This destroys the radicals, and terminates the reaction.
H. + H.  H2
H2
Cl. + Cl.  Cl2
Cl2
H. + Cl.  HCl
HCl
- The overall effect of the reaction is to combine hydrogen and chlorine:
H2(g) + Cl2(g)  2HCl (aq)
2HCl (aq)
- Free radical reactions usually occur in the gas phase, are initiated by heat or light, occur very fast, and can result in a variety of products.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home
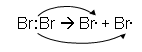


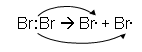

 H+ + :Cl-
H+ + :Cl-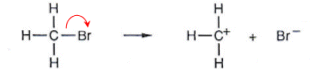



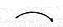 shows the movement of one electron.
shows the movement of one electron.
 2Cl.
2Cl.
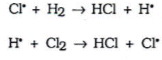
 H2
H2 Cl2
Cl2 HCl
HCl 2HCl (aq)
2HCl (aq)