
Whatís in the Air?
- The atmosphere extends to around 100km above the earthís surface.
- The atmosphere is divided vertically into four regions- the troposphere, the stratosphere, the mesosphere and finally the thermosphere.
- There is a negative correlation between height and density; i.e. the higher you go, the less dense the atmosphere is.
- The temperature decreases in the troposphere, but is constant at -57oC in the stratosphere, before decreasing in temperature in the mesosphere, and then increasing in temperature beyond the mesopause in the thermosphere.
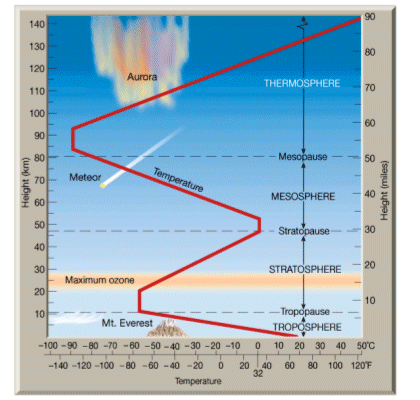
- The atmosphere is a mixture of many gases. 90% of all molecules in the atmosphere are found in the troposphere. Mixing of these gases is easy in the troposphere because of convection currents.
- The low temperatures in the stratosphere mean that vertical movement is difficult. Horizontal circulation occurs in the stratosphere.
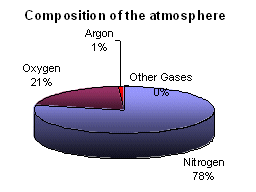
-
The pie chart shows the main constituent gases of the atmosphere. In addition to these gases, there are also trace gases.
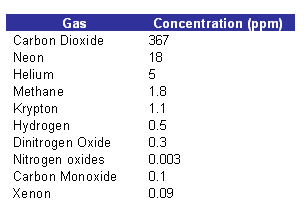
-
The concentrations of the gases in the table above are measured in parts per million. This measure is adequate when small concentrations are involved. 35 ppm represents a percentage concentration of 0.035%.
- The atmosphere hasnít always been the same, it has developed over billions of years.
- The first atmosphere was lost altogether during the turbulent period in the early stages of our solar system.
- The next atmosphere contained mainly carbon dioxide, methane, and ammonia, which came from stores underground.
- 3,000 million years ago the first organisms were born, and these were able to use the suns light for their energy. These organisms began to produce oxygen.
- Not much of this oxygen ended up in the atmosphere during the first 1000 million years, as it was used up in chemical reactions such as the oxidation of iron and sulphur in the earthís crust.
- When the oxygen concentration reached 10%, there was enough for the first animals.
- Eventually there was so much respiration and other processes requiring oxygen, that oxygen was being removed as fast as it was formed.
- Since then the oxygen concentration has remained at around 21%.
- In the table previously, all of the gases are produced by natural processes.
- However, human activities add more gases to the atmosphere, some are already present and we are just increasing their concentration.
- Gases always mix completely in the atmosphere, this is helped by air currents.
- Eventually, pollutant gases will spread throughout the atmosphere.
- Atmospheric pollution is a global problem; two of the major troubles are the destruction of the ozone layer, and the excessive Greenhouse Effect.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home




