
The OH Group
- The OH (hydroxide) group can occur in three different environments within organic molecules: alcohols, acids, and phenols.
Alcohols
- There are three classifications of alcohols, each one dependant on the position of the OH group:
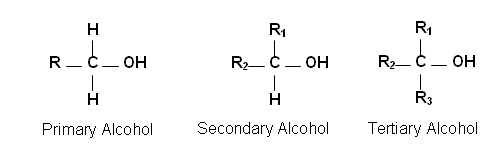
- Primary alcohols are attached to a carbon with only one R-group (carbon chain or other atoms other than hydrogen).
- Secondary alcohols are attached to a carbon with two R-groups.
- Tertiary alcohols are attached carbon with three R-groups.
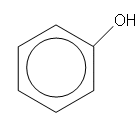
Phenols
- These occur when an OH group is attached to a benzene ring. Phenols behave very differently to alcohols.
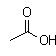
Carboxylic acids
- Carboxylic acids contain a COOH group.
Acidic Properties
- The –OH group can react with water:
R-OH + H2O R-O- + H3O+
R-O- + H3O+
- Water itself can also do this and so is acting as a weak acid (donating H+ ions):
H-OH + H2O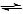 H-O- + H3O+
H-O- + H3O+
- So ethanol and water act as weak acids, but with ethanol the position of equilibrium of the reaction lies further to the left than it does in water, thus ethanol is a weaker acid.
- With phenol, the position of equilibrium lies further to the right, as it is a stronger acid.
- Carboxylic acids are even stronger acids than phenols; however they are still only weak acids.
- The running order of acid strength is:
Ethanol < Water < Phenol < Carboxylic Acids
- The stability of the R-O- formed decides how strong the acid is. If the –ve charge on the oxygen can be shared with other atoms, then it will be more stable, thus more of it will be made.
- No sharing can occur in alcohols; however in phenols and carboxylic acids, sharing can occur as the electrons spread out around the whole ion (delocalised electrons).
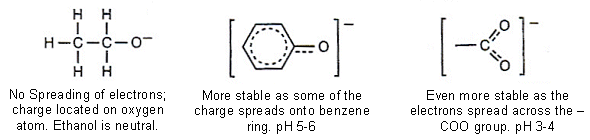
- Phenols and carboxylic acids react with bases:
- Only carboxylic acids react with carbonates to produce carbon dioxide.
- Carboxylic acids and phenols react with NaOH(aq) to form their sodium salts.
- Alcohols react with neither NaOH(aq) nor any of the carbonates.
Oxidation of Alcohols
- Primary alcohols can be oxidised to make aldehydes, which can then be oxidised to carboxylic acids.
- Secondary alcohols are oxidised to ketones.
- Tertiary alcohols cannot be oxidised.
- Phenols and carboxylic acids can’t be oxidised as they don’t have a hydrogen atom on the carbon atom to which the OH group is attached.
- The –OH group is oxidised by strong oxidising agents such as acidified potassium dichromate (vi):

- The orange dichromate ion is reduced to green Cr3+.
- Two hydrogen atoms are being removed from the molecule (one attached to the carbon and the other from the –OH group).
- The product is a Carbonyl group (either a ketone or aldehyde).
- The product depends upon the kind of alcohol you start with.
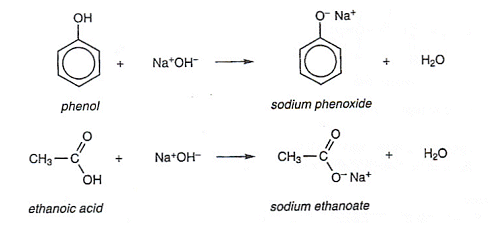
Aldehydes and Ketones
- Aldehydes and Ketones contain a carbonyl group:
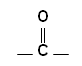
- In an aldehyde, the carbonyl group is at the end of the alkane chain:
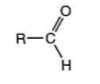
- Aldehydes are named using the suffix “-al”:
CH3 –CHO (ethanal)
- In a ketone, the carbonyl group is inside the alkyl chain:
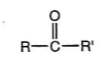
- Ketones are named using the suffix “-one”:
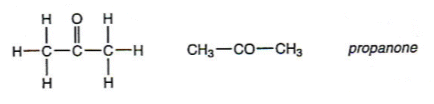
The Reactions
- Primary alcohols are oxidised to aldehydes and then carboxylic acids. For example, ethanol is oxidised to ethanal, which is then oxidised to ethanoic acid:
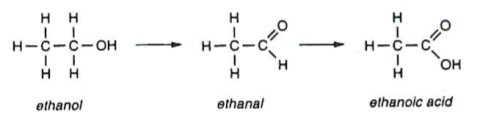
- Secondary alcohols are oxidised to ketones. For example, propan-2-ol is oxidised to propanone:
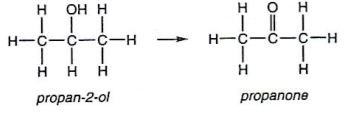
- Tertiary alcohols are difficult to oxidise, as they do not have a hydrogen atom on the carbon atom to which the –OH is attached.
Dehydration of alcohols
- Alcohols can lose a molecule of water to become an alkene.
- Propene is formed when propan-1-ol vapour is passed over a hot catalyst of alumina (300oC).
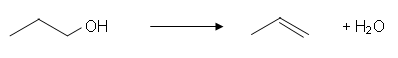
- It is a dehydration reaction because it involves the removal of a water molecule from a molecule of the reactant.
- Dehydration can also occur when heated with concentrated sulphuric acid.
- Dehydration is an example of an elimination reaction (reverse of an addition reaction).
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry