
The Synthesis of Salicylic acid and Aspirin
- Drugs can be hard to obtain when needed. In the case of some medicines, the supply may be seasonal, depend on weather conditions and even be liable to contamination.
- Once the chemical structure of the drug is known, chemists begin to look for ways to produce it artificially.
- For complex drugs, scientists search for a compound already known with a similar structure to the drug, which can be modified.
- The active drug in willow bark is 2-hydroxybenzoic acid.
- The compound phenol was already known in the chemical industry at the time when aspirin was being developed.
-
Phenol’s chemical structure only differed from that of 2-hydroxybenzoic acid by one functional group.
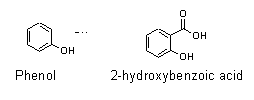
- The problem of synthesis was to introduce the new functional group to the molecule without disrupting the rest of its structure.
- In this case carbon dioxide could be used to combine directly with the phenol to produce 2-hydroxybenzoic acid.
- This procedure is known as the Kolbe synthesis, and a German scientist (Hoffmann) developed an industrial version of it.
- The new drug was used by many for curing fevers and suppressing pain; however reports began to accumulate claiming that the drug had irritating side affects on the gullet and stomach.
- Scientists now had a new problem; was it possible to develop the drug to reduce the irritating effects, whilst still retaining the useful ones?
- Hoffman made many modifications to the structure of 2-hyroxybenzoic acid, eventually synthesising 2-ethanoylhydroxybenzoic acid, which became known as aspirin.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry