
Transition Metals
- A transition metal is an element which forms at least one ion with a partially filled sub-shell of d electrons.
- The energy between the s sub-shell and d sub-shell are so similar, that electrons from both can be involved in bonding.
- Not all of the elements within the d-block are transition metals, for example zinc has a complete d sub-shell, and so it does not share the similar characteristics of a typical transition metal.
Physical properties of transition metals
- Transition metals are all reasonably similar to each other, but show distinct differences between metals from the s-block.
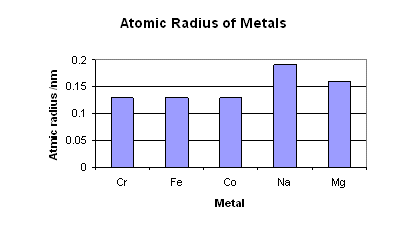 The atomic radii of the transition metals in the same period show very little differences. This is because each extra electron that the proceeding element has is placed in the 3d shell, rather than the outer shell. After each successive element one extra proton is added.
The atomic radii of the transition metals in the same period show very little differences. This is because each extra electron that the proceeding element has is placed in the 3d shell, rather than the outer shell. After each successive element one extra proton is added.
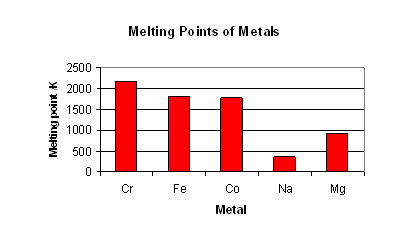
The melting-points of the transition metals are high due to the 3d electrons being available for metallic bonding.
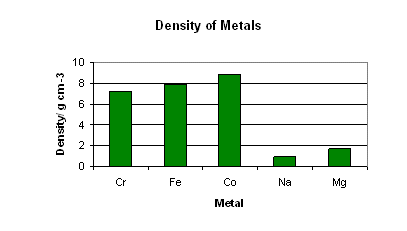
The densities of the transition metals are high for the same reason as the high boiling points.
- Transition metals are all dense metals with high melting and boiling points.
- They are often hard and durable, with high tensile strength and good mechanical properties.
- These properties are the result of metallic bonding between the atoms in the metal lattice.
Metallic Bonding
- The strong forces which act between the separate atoms within a metal are known as metallic bonds.
-
The diagram below explains why metals act as they do:
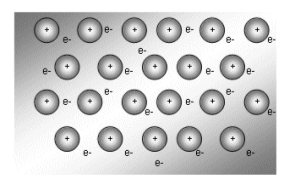
- The positive ions are arranged in a regular spaced lattice shape. The outer shell electrons move freely through this lattice. The free electrons are often described as a “cloud” or “sea” of electrons.
- Each positively charged ion is attracted to the “sea” of negative electrons.
- The electrostatic attraction binds the entire structure together as one unit.
- A particular electron in a metal does not belong to one of the positive ions, but is attracted to all of them. These electrons are described as delocalised.
- The strength of the metallic bonding depends upon the number of electrons. Therefore magnesium (two outer electrons) has stronger metallic bonding than sodium (one outer electron).
- This strong electrostatic attraction is why metals have high boiling/melting points, and are dense strong materials.
- They are good conductors of electricity due to the delocalised electrons that are mobile.
- Transition metals are able to release electrons for bonding from both their inner and outer sub-shells (s shell and d shell).
- This means that there are more electrons holding the structure together, so the metallic bonding is stronger.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry