
Rusting
- Most metals are found in the Earthís crust as an oxide or other compound.
- This is because they are energetically more stable as an oxide than they are as a metal.
- To extract the metal from its ore requires reduction; this uses a great deal of energy.
- Eventually the metal is oxidised, or corroded to form its ore.
- Iron corrodes to form rust; i.e. it rusts.
- This is a problem for car owners, as cars are made from steel.
- Cars rust because the steel that they are made from reacts with oxygen and water in the atmosphere.
- The iron rusts to form a hydrated form of iron(III) oxide (Fe2O3.xH2O).
- This oxide is permeable to air and does not form a protective layer on the metalís surface; this leads to the corrosion of the metal beneath the layer of rust.
-
Iron and steel will rust when they come into contact with moist air; however the rate at which the rusting takes place depends upon many factors, such as:
- The composition of the metal.
- Impurities in the iron/steel surface.
- Electrolytes/solutions in contact with the metal.
- Presence of acids.
- The availability of dissolved oxygen in the solution.
What happens to steel and iron during rusting?
-
Rusting is an electrochemical process; electrochemical cells are established in different regions of the metal surface. Some areas act as anodic (electron releasing) cells, whilst others act as cathodic (electron accepting) cells.
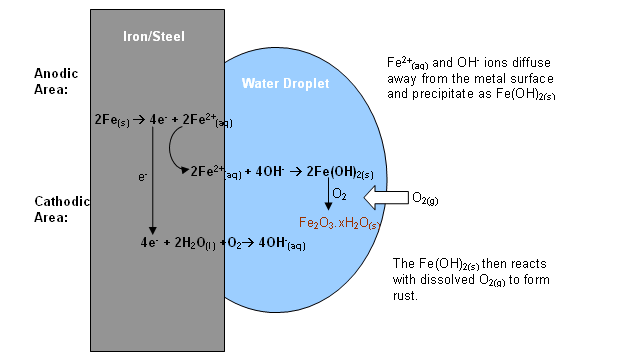
- The parts of the metal surface where dissolved oxygen concentration is high act as Cathodic areas; regions with a limited oxygen supply act as anodic areas.
- Therefore, rusting is always greatest at the centre of a water drop, or under a layer of paint, as these are the anodic regions.
- Pits are formed here when the iron has dissolved away.
- Rust then forms in these pits when the Fe2+ and OH- ions diffuse away from the surface and react in a secondary process within the solution.
- Acidic conditions accelerate rusting by encouraging iron to dissolve away from the metal surface.
- Ionic compounds, e.g. sodium chloride from road grit, promote rusting by increasing the conductivity of the water.
Anodic Reaction:
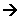 Fe2+(aq) + 2e-
Fe2+(aq) + 2e-Cathodic Reaction:
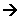 4OH-(aq)
4OH-(aq)Overall Reaction:
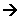 2Fe(OH)2(s)
2Fe(OH)2(s)- The green precipitate formed is then oxidised further to form a brown precipitate of Fe2O3XH2O.
Preventing Corrosion
- The simplest way to protect iron and steel from rust is to provide a physical barrier between the metal and the atmosphere. This barrier could be oil/grease or paint.
- A barrier made from an organic polymer is often chosen nowadays; the steel is coated with a plastic film. This provides a colourful and effective way of protecting steel from the environment.
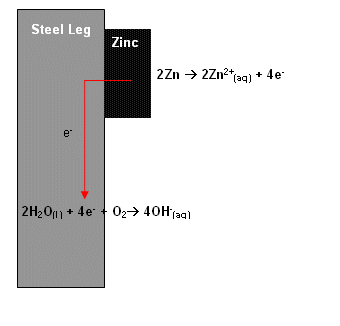
- Another solution to corrosion is galvanisation. This is where the iron/steel is coated with a thin layer of another metal.
- Zinc is an ideal metal, as it is more reactive than iron (stronger reducing agent; gives up electrons more readily), and so is corroded in preference to iron. The zinc layer will be protected from corrosion by a firmly adhering layer of zinc oxide.
-
Zinc is being used as a sacrificial metal. Zinc is widely used on North Sea oil rigs to protect them from corrosion. The corrosion is transferred from the valuable complex steel structure to a readily available lump of metal.
Stainless Steel
- Much of the steel you come into contact with around the home, particularly those that come into regular contact with water (e.g. cutlery) are made of stainless steel.
- Stainless steel was developed by a Sheffield chemist called Harry Brearley (1913). He was investigating the rapid wear of rifle barrels and decided to make a steel alloy with a high level of chromium to see if this would prolong their usage.
- He discovered that his steel would not dissolve in acids, they also remained shiny when left for long periods of time.
- Brearley decided that his new steel would make an excellent material from which to produce cutlery.
- The reason why stainless steel doesnít rust, is because it forms a protective outer layer of chromium oxide (Cr2O3).
- Unlike rust, the oxide is not hydrated and adheres closely to the metal surface.
- It is only a few nanometres thick and so is invisible to the eye, it does, however, leave the metal with a shiny finish.
- If the surface film is scratched, it quickly reforms and restores the protection.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry