
Predicting the direction of Redox Reactions
- Redox reactions involve the transfer of electrons.
- The oxidising agent is reduced (i.e. it gains electrons; it is the “electron thief”).
- The reducing agent is oxidised (i.e. it loses electrons; it is the “electron donator”).
- Redox reactions can be written in two half reactions; one reaction producing the electrons and the other reaction accepting them.
-
For example, when Zinc is added to copper sulphate, the following reaction occurs:
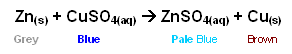
- In addition to just the colour change, the reaction is also exothermic and so results in an increase in temperature.
-
The reaction can be simplified; the sulphate ion is a spectator ion and so can be removed.
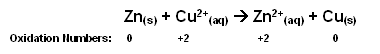
- From the oxidation numbers, it is plain to see that the zinc is being oxidised (increase in oxidation number) and the copper is being reduced (decrease in oxidation number).
-
The two half equations are:
Cu2+(s) + 2e- Cu(aq) (reduction)
Cu(aq) (reduction)
Zn(s) Zn2+(aq) + 2e- (oxidation)
Zn2+(aq) + 2e- (oxidation)
- The reverse reaction of this can not occur; if copper is added to zinc sulphate solution, nothing will happen.
-
However, if copper is added to silver nitrate solution, the copper does react:
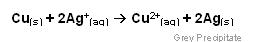
-
The half equations are:
2Ag+(s) + 2e- 2Ag(s) (reduction)
2Ag(s) (reduction)
Cu(s) Cu2+(aq) + 2e- (oxidation)
Cu2+(aq) + 2e- (oxidation)
- Once again the reverse reaction cannot occur.
-
However; as you can see from the two previous reactions, the individual half reactions are reversible:
Cu(s)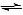 Cu2+(aq) + 2e-
Cu2+(aq) + 2e-
- The actual direction that the reaction takes depends upon what it is reacting with. Copper ions are reduced by zinc (the zinc supplies electrons); however copper is oxidised by silver ions (the copper provides electrons).
- Copper atoms don’t transfer electrons to zinc ions, and silver atoms don’t transfer electrons to copper ions.
- Something must control the direction of electron transfer; to find out which way the electrons are transferred requires study of the individual half-equations, which can be done by separating the half-reactions using an electrochemical cell.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry