
The d-Block Elements
- The d-Block consists of three series in Periods 4, 5, and 6. Each series contains ten elements.
-
The special electronic configurations of the d-block elements results in them having different characteristics to elements in other parts of the periodic table.
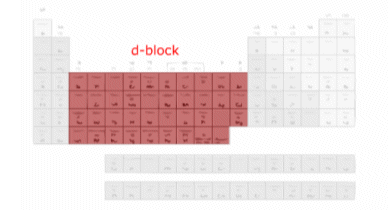
- The differences between the elements within a group in the d-block are much less sharp than the differences between the elements in p-block or s-block group elements.
- The similarities across a period are also much greater.
- Thus we can discuss the d-block as a group of elements with very similar properties.
Electronic Configuration of the d-block elements
- With each successive element across a period, there is one extra proton and one more electron (compared to previous element).
-
Each extra electron enters the 3d shell (hence the elements are in the d-block).
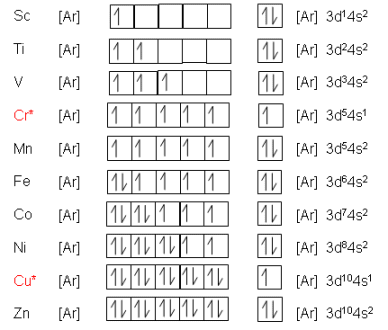
- All the d-block elements essentially have the same outer electron arrangement as each other, as do the elements in vertical groups.
- Also, they do not differ by a complete electron shell as do the elements in a group; they differ by having one more electron in the inner complete 3d sub-shell.
- This is why their properties are similar.
*Copper and Chromium
- In the ground state of an atom, the electrons are always arranged to give the lowest energy value.
- Electrons repel each other (both have negative charge), so a lower energy is achieved by placing the electrons singly in orbitals than if they are paired.
- The energies of 3d and 4s orbitals are very close together in period four. It works out that with chromium and copper, putting the two electrons in the 4s orbital would result in a higher energy, thus they fill the 3d orbital first.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry