
Complex Formation
- A complex is made up of a central metal atom/ion surrounded by negatively charged ions or neutral molecules possessing a lone pair of electrons (ligands).
- A complex may be charged or uncharged, if it is charged it is called a complex ion, for example [Fe(H2O)6]3+.
- The overall charge is the sum of the charge on the central metal ion and the charges on the ligands.
- A simple way of looking at the bonding in a complex, is looking at the bonds as being co-ordinating bonds from the ligands (i.e. the ligands give up their pair of electrons to form a dative bond).
- The co-ordination number is the number of co-ordinating (dative) bonds to the central metal ion; the most common co-ordination numbers are 6 and 4.
-
The shape of the complex is determined by the co-ordination number:

- Most complexes form the shapes above; however, there are some exceptions, for example in the [Cu(H2O)6]2+ Ion, four of the water ligands are held more strongly than the other two; this results in a distorted tetrahedral shape.
Naming Complexes
- Give the number of ligands around the central complex ion using the prefixes mono-, di-, tri-, tetra-, penta-, hexa-…..
- Identify the ligands (alphabetical order if more than one type) using the ending –o for ions (e.g. fluoro). Neutral ligands keep their name (except H2O which is aqua and NH3 which is ammine).
- Name the central metal ion; if the overall charge on the ligand is positive or neutral, use the English name, if it has a negative chage, use the latinised name (usually add suffix –ate).
- Indicate the oxidation number of the central metal in brackets.
For example:
[Cr(H2O)6]3+ hexaaquachromium(iii) ion.
[Fe(CN)6]3- hexancyanoferrate(iii) ion.
[CuCl4]2- tetrachlorocuprate(ii) ion.
- The above complexes are all formed from monodentate ligands; the word "dentate" derives from the Latin meaning of "tooth".
- Some ligands have more than one site that can act as a ligand; these are known as polydentate ligands.
-
1,2 di-amino-ethane (ethylenediamine) is an example of a bidentate ligand:

- Each nitrogen has a lone pair which can form a dative bond to the metal ion.
- The name of this ligand is often abbreviated to “en”, for example [Cr(en3)]3+ .
- The metal ion is held in a five membered ring by the dative bonds (like claws of a crab holding the ion). The ring is called a chelate ring from the Greek meaning word meaning “crab”.
-
An important polydentate ligand is edta4- (edta is an abbreviation of the older name for the molecule, ethylenediaminetetraacetic acid):

- The edta molecule has six lone pairs of electrons and so is a hexidentate ligand.
- It complexes as a edta4- ion.
- The molecule surrounds the metal ion and traps it within it.
Stability of complexes
- Ligands form bonds of different strengths with metal ions and so form complex with varied stabilities.
- The stability constant Kstab is used to compare the stability of different complexes.
-
They are equilibrium constants for the formation of a complex made by replacing water with the new ligand, for example:
[Ni(H2O)6]2+(aq) + 6NH3(aq) [Ni(NH3)6]2+(aq) + 6H2O(l)
[Ni(NH3)6]2+(aq) + 6H2O(l)
- The greater the stability constant, the further to the right the position of equilibrium and therefore the more stable the complex.
-
The stability constants are usually very large numbers and so the values are quoted on a logarithmic scale.

- The stability constants for the poldentate ligands are much larger than for monodentate ligands.
- This is because the forming of the complex results in a large increase in entropy.
- In the case of edta4- , a single ligand can replace up to six water molecules; this increases the number of single particles present, thus increasing the entropy.
Competition between Ligands
- Some ligands form stronger bonds than others with a particular metal ion. In this case, the better ligand will displace the poorer one.
- For example, copper(II) sulphate dissolved in water forms the hexaaquacopper(II) ion, [Cu(H2O)6]2+, which is why the solution is pale blue in colour.
- Addition of chloride ions in high concentrations leads to a stepwise displacement of H2O ligands by Cl- ligands:
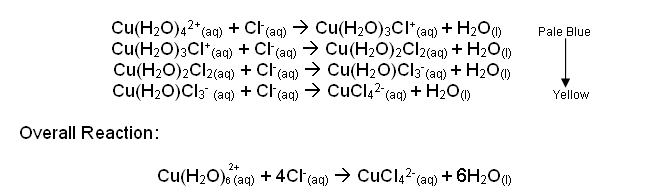
- For each reaction, the equilibrium lies slightly to the right, implying that Cl- is a better ligand than H2O.
- Ammonia is an even better ligand than Cl- and it will displace both water and chloride ions from the metal ion.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry