
Catalytic Activity of Transition Metals
- A catalyst is a substance which changes the rate of a reaction without being used up itself in the process.
- Catalysts are extremely important substances; without them, many industrial reactions would be commercially uneconomical.
- Transition metals make good catalysts; they may be divided into two groups; homogenous and heterogeneous catalysts.
Homogeneous Catalysts
- Persulphate ions, S2O82-, oxidise iodide ions:
S2O82-(aq) + 2I- (aq) 2SO42- (aq) + I2(aq)
2SO42- (aq) + I2(aq)
- The above reaction occurs very slowly at room temperature, and so a catalyst is used.
- The catalyst used is iron(II); it is believed that the catalyst reaction takes place in two steps.
- Firstly, the persulphate ions oxidise iron(II) to iron(III):
2Fe2+ (aq) + S2O82- (aq) 2SO42- (aq) + 2Fe3+ (aq)
2SO42- (aq) + 2Fe3+ (aq)
- Then the Fe3+ oxidises I- to I2:
2Fe3+(aq) + 2I-(aq) 2Fe2+(aq) + I2(aq)
2Fe2+(aq) + I2(aq) - So, in this reaction the Fe2+ ions have been regenerated; the activation of the reaction is also lower with the Fe2+, so it is acting as a catalyst.
- Part of the reason for the activation energy for the catalysed reaction being lower, is that the uncatalysed reaction takes place between two negatively charged ions; whereas both steps of the catalysed reaction involve reactions between two oppositely charged ions.
- Looking at the
 values:
values:
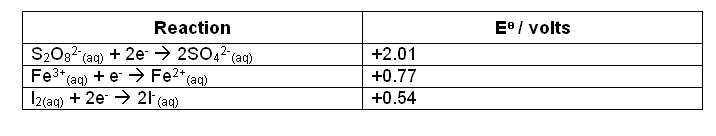
- It is obvious from the reaction that the catalyst has to be the correct metal, for it must have an
 in between that of the reduction of persulphate and the oxidation of iodide ions.
in between that of the reduction of persulphate and the oxidation of iodide ions.
- Transition metals are frequently used to catalyse redox reactions like the one above. This is because they have variable oxidation numbers and can therefore act as a temporary “warehouse” of electrons; at first they act as a reducing agent, giving up electrons, then they act as an oxidising agent, receiving electrons.
Heterogeneous Catalysts
- The ability of transition metals to accept electrons from ligands (due to unfilled d-orbitals) play a role in catalysis.
- Gases are adsorbed onto the surface of the metal (form weak bonds with the metal ions); this facilitates the reaction in a number of possible ways:
- The reactants are in a much closer proximity when adsorbed onto the metal surface, therefore the concentration of the reactants increases, thus the rate of reaction does too.
- The bonds that form onto the catalyst may withdraw electron density from the molecules internal bonds, thus weakening them.
- The adsorbed reactants may be adsorbed onto the metal surface in just the right orientation for the reaction to occur.
- The strength of the adsorption of the gases is essential; if the bonds are too strong, then the products will remain fixed to the catalyst, preventing further catalysis (catalyst poisoning).
- However, with a successful catalyst, the products are desorbed from the catalyst surface, leaving the active sites available for more gases.
- Some of the metals that are good catalysts are rare and expensive, and so they are often supported on a ceramic surface, so that every atom is available for catalysis.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry