
Iron Extraction
- To make iron, it is first necessary to mine iron ore.
- Iron ore is simply rock with a high concentration of iron within it. Usually the ores are mixed into rocks which contain silica.
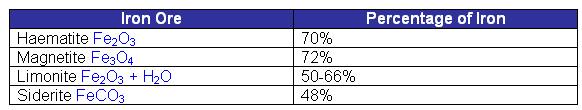
- From the table above, it is evident that the iron ores contain oxygen.
- In order to make the iron from the ore, the oxygen must first be removed. This can be achieved using a blast furnace in which a series of reduction reactions occur.
- Ores of iron greater than 5mm can be placed straight into the blast furnace; however these are in short supply and so the iron first goes through agglomerating processes (fuse together the smaller lumps of ore) such as sintering or pelletising. These processes involve mixing together the iron with controlled quantities of fluxes.
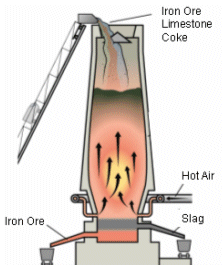
- Iron, limestone and coke are fed into the top of the blast furnace. The limestone is melted to become the slag, which removes impurities such as sulphur.
- The main fuel used in the blast furnace is coke. This is made by heating carbon in the absence of oxygen. The coke is burnt in heated air (1,100 K to 1,600K) and then blown into the furnace at the bottom. The coke reacts with oxygen in the hot air blast to form carbon monoxide:
2C(s) + O2(g) 2CO(g)
2CO(g)
- Hydrocarbons can also be used as a fuel, and these react similarly to produce carbon monoxide and hydrogen.
CH4(g) + 0.5O2(g) CO(g) + 2H2(g)
CO(g) + 2H2(g)
- The gases move up the blast furnace where they reduce the iron oxides (at different temperatures):
Fe2O3(s) + 3CO(g) 3CO2(g) + 2Fe(s)
3CO2(g) + 2Fe(s)
Fe2O3(s) + 3C(s) 2Fe(s)+ 3CO(g)
2Fe(s)+ 3CO(g)
- The products formed from the reactions above begin to melt and trickle down to the bottom of the blast furnace.
- The limestone also descends to the bottom of the blast furnace where it is thermally decomposed:
CaCO3(s) CaO(s) + CO2(g)
CaO(s) + CO2(g)
- The basic calcium oxide produced in this reaction then goes on to react with acidic impurities in the iron:
FeS(s) + CaO(s) + C(s) CaS(s) + FeO(s) + CO(s)
CaS(s) + FeO(s) + CO(s)
CaO(s) + SiO2(g) CaSiO3(s)
CaSiO3(s)
- The CaS and CaSiO3 become the slag, which also contains other impurities such as MgO, CaO, SiO2and Al2O3. The liquid slag is less dense than iron and so it floats on top of the molten iron at the bottom of the furnace.
- The molten iron and liquid slag are removed from the furnace through the tap holes at the base.
- The slag can be used to make bi-products such as cement. The hot gases produced are cleaned and are then burned as a fuel in the hot blast stoves that are used to preheat the air that is blown into the furnace.
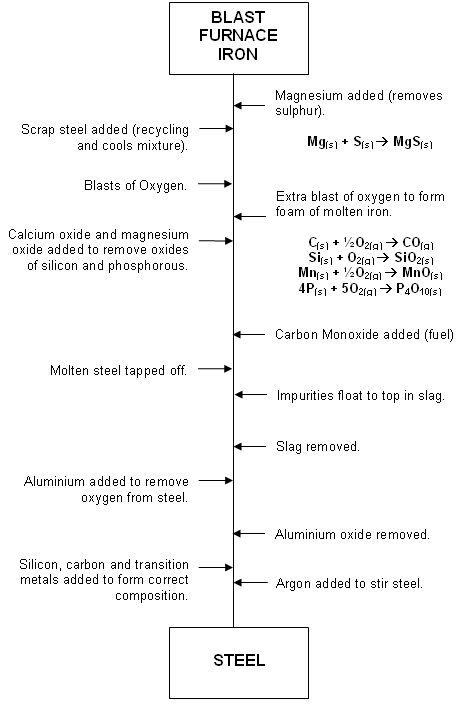
The Basic Oxygen Steel making (BOS) Process
- In the UK, steel is made using the BOS process. Around 300 tonnes of steel can be made in one forty minute batch using this process.
- The iron made from the Blast Furnace is transported to the BOS vessel where it is mixed with controlled amounts of scrap steel.
- A water-cooled oxygen lance is lowered into the furnace providing a blast of high purity oxygen (blasted at twice the speed of sound). The blast of oxygen removes some of the impurities:
2C(s) + O2(g) 2CO(g)
2CO(g)
Si(s) + O2(g) SiO2(s)
SiO2(s)
2Mn(s) + O2(g) 2MnO(s)
2MnO(s)
4P(s) + 5O2(g) P2O5(g)
P2O5(g)
- The products from the above reactions are removed either in liquid state in the slag, or gaseous state at the top.
- The composition of the steel is now adjusted by adding other elements such as manganese, carbon, nickel, chromium, molybdenum, cobalt and tungsten.
The Uses and Composition of Steels
- The amount of carbon in steel ranges from 0.1% to 1.5% giving steel different properties. Steel composed of less than 0.25% carbon is called mild steel.
- Mild steel is cheap, strong and easily shaped, and as so is the main metal for construction (used to make bridges, buildings, ships and vehicles.
- Steels with more than 0.5% carbon are stronger than mild steel; however the steel is more brittle, and so is used to make tools.
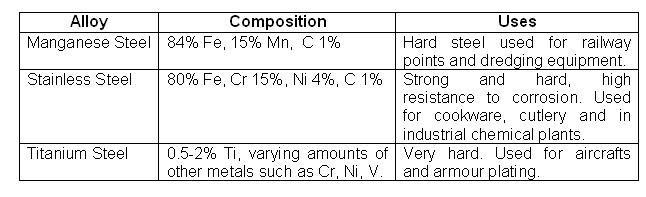
- Desirable properties of steel can also be attained by alloying it with other metals.
The Basic Oxygen Steel making (BOS) Process Summary
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry