
Proteins
- Proteins are very important for living organisms;
- They act as hormones (e.g. insulin).
- They form muscle fibres.
- Proteins form many cell structures (protein carriers etc).
- They are important structural molecules (hair, nails and feather are all made from proteins).
- Enzymes are made from proteins.
- They form many binding proteins (haemoglobin etc).
- There are only twenty
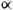 -amino acids in the world. These twenty amino acids are joined together in different sequences to form every protein present in living organisms.
-amino acids in the world. These twenty amino acids are joined together in different sequences to form every protein present in living organisms.
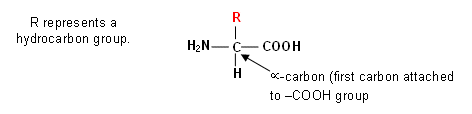
- The twenty
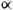 -amino acids have the same general structure (above); however they have different side-chains (R-group). For example,
-amino acids have the same general structure (above); however they have different side-chains (R-group). For example, 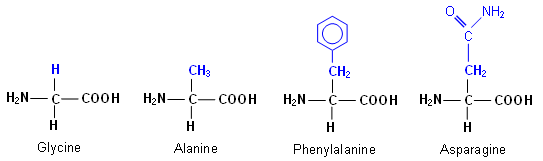
- Proteins are constantly being replaced in the human body; this is evident as our hair and nail continue to grow even after being cut.
- It is also important that some hormones and enzymes are produced when they are needed, and destroyed when they are no longer needed.
- We replace our proteins with those that we consume; however we aren’t required to eat the proteins that we need (if that were the case we would have to eat human hair in order to grow our own).
- Instead our bodies break down the proteins into amino acids; eight of which are essential amino acids (our bodies cannot make them), the other twelve our bodies can synthesise from carbohydrates and other amino acids.
- The amino acids are then re-assembled to make our own collection of proteins, which are unique for every living organism (many may be the same for living organisms, but they are not all the same).
- There are millions upon millions of proteins around the world, and these are all made from the twenty different amino acids.
- What makes the proteins different from each other are the order in which the amino acids are joined; the primary structure of the protein.
Synthesising Peptides
- Amino acids join together to form polypeptides, which make proteins.
- The reaction is an example of a condensation reaction, as one amino acid joins onto the next forming a larger molecule with the loss of a smaller molecule.
- The amino acids join together when the carboxylic acid group on one amino acid joins on to the amino group on the next (a molecule of water is lost each time).
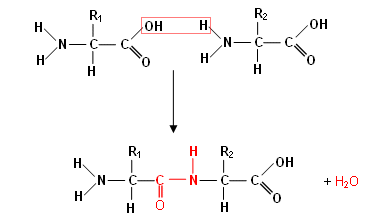
- The –CONH– (red) is called a peptide group; this may look familiar, in designer polymers you came across this group in nylon; however it is referred to as an amide group.
- In the molecule above, the two peptides have joined together to form a dipeptide; many peptides join together to form a polypeptide.
- Chemists cannot make amino acids react directly together. Instead, they make the COOH group more reactive by converting it into an acid chloride.
- Chemists also have to look at another property of amino acids when synthesising proteins.
- Amino acids are not flat molecules; they have a three-dimensional shape, based upon a tetrahedron. This means that they exist in two isometric forms (except glycine) known as D or L isomers.
- Proteins are synthesised only from L isomers.
- Cells are able to synthesise proteins directly from amino acids.
- Chemists are using bacterial cells and yeast cells to make proteins for them, as in many ways it is better than using traditional techniques.
- In order to synthesise a protein, chemists need:
- A set of instructions for the protein (giving you the primary structure).
- Supplies of pure amino acids (separated).
- A way of making the amino and carboxylic acid groups more reactive.
Hydrolysing Polypeptides
- As polypeptides are held together by peptide linkages, which are the same as amide linkages, they can be hydrolysed in the same way as polyamides.
- In the body, enzymes would be used to help hydrolyse the peptide bond; however the peptide bonds can be broken in a reflux reaction with hydrochloric acid.
- Aspartame is an artificial sweetener which can be used as a useful substitute for a protein to demonstrate hydrolysis.
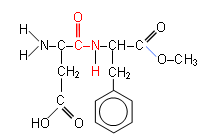
- When Aspartame is heated in the presence of concentrated hydrochloric acid, the peptide bond is hydrolysed in addition to the ester linkage.
- This results in the formation of aspartic acid, phenylalanine and methanol:
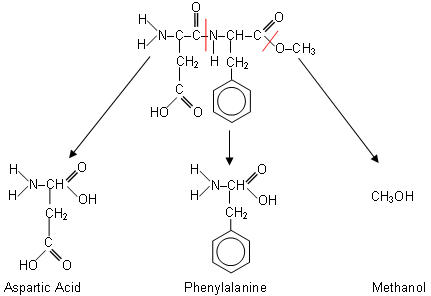
- This is the reason for the expiry date on diet coke; diet coke contains aspartame and also phosphoric acid (flavour enhancer). The phosphoric enhancer catalyses the hydrolysis of aspartame and so after a length of time, the diet coke loses its sweet flavour as the sweetener is hydrolysed..
- These products (except methanol, because it evaporates too fast) can be identified using chromatography.
- Chromatography works on the basis that some compounds are more soluble than others, and so when left to move with a solvent up chromatography paper, the compounds will leave the solvent at different points (i.e. the more soluble the compounds, the further they will travel up the chromatography paper).
- In this experiment the solvent is a mixture of butan-1-ol and ethanoic acid.
Technique
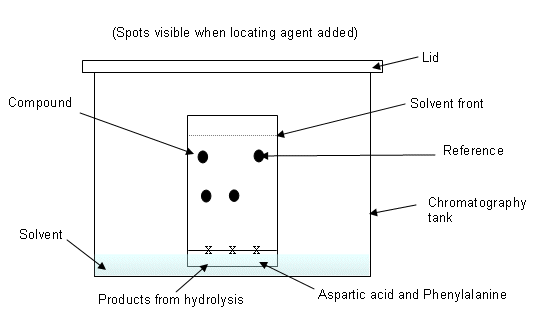
- Draw a pencil line approximately 1cm from the bottom of the paper. Mark a cross and produce a concentrated spot of the compounds to be used by repeated application followed by drying.
- Mark a reference spot of aspartic acid, and phenylalanine.
- Mark a spot of the product from the hydrolysis of aspartame.
- Place in chromatography tank; ensure that the spots are above the solvent (butan-1-ol and ethanoic acid).
- Cover with lid.
- Allow time for the solvent to run up the chromatography plate, so it reaches just below the end of the paper.
- Use locating agent to find spots (ninhydrin), and then heat using a hair dryer.
- Compare the products from the hydrolysis of aspartame with the reference compounds.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home
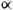 -amino acids in the world. These twenty amino acids are joined together in different sequences to form every protein present in living organisms.
-amino acids in the world. These twenty amino acids are joined together in different sequences to form every protein present in living organisms.

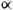 -amino acids have the same general structure (above); however they have different side-chains (R-group). For example,
-amino acids have the same general structure (above); however they have different side-chains (R-group). For example, 




