
Nuclear Magnetic Resonance (N.M.R.) Spectroscopy
The basis of N.M.R.
- Nuclear magnetic resonance spectroscopy is an extremely useful technique for organic chemists.
- N.m.r. provides chemists with detailed information about the nuclei of hydrogen atoms; the nuclei behave differently in different environments, thus n.m.r. can tell us what the environments are and how many hydrogen atoms are within them.
- N.m.r. can be used to find out about any nuclei that has an odd number of protons + neutrons; in addition to 1H nuclei, 13C, 19F and 31P are also used for n.m.r.
- When nuclei are placed in a strong magnetic field, they act like small magnets; they can either align themselves with the magnetic field, or against it (requiring more energy).
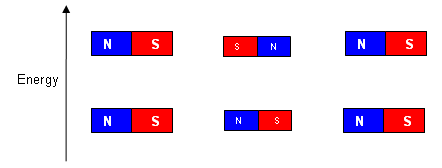
- More than 50% of the nuclei align themselves with the magnetic field (lower energy).
- Like when electrons are promoted, it requires a definite amount of energy to move the nuclei up to a higher energy level (aligned against the magnetic field); this amount of energy is different for the hydrogen nuclei in different environments.
- The energy that is needed to move the nuclei up to a higher energy level corresponds to that of radio waves.
- The energy required depends upon the strength of the overall magnetic field on the nuclei.
- The strength of the magnetic field not only corresponds to the strength of the instrument’s magnetic field, but it also depends upon the magnetic fields of the groups surrounding the hydrogen nuclei.
- The electrons associated with neighbouring atoms have a small magnetic field themselves, which usually opposes the external magnetic field. The overall field that is experienced by the proton is therefore smaller than the external field; the small change associated with the value of the local field from the surrounding part of the molecule.
- This means that for every different kind of molecular environment, there is a different magnetic field.
- Thus in different molecular environments, the energy gap between the lower and higher energies of the nuclei changes; therefore in different environments the hydrogen nuclei absorb different frequencies of radiation.
The N.M.R. Machine
- The n.m.r. machine comprises of a magnet (produces strong magnetic field), a radio-frequency source, a detector and a recorder.
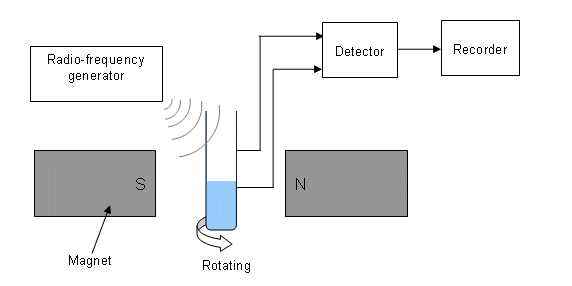
- The samples are dissolved in solvents which contain no hydrogen atoms (e.g. CCl4) or that have the hydrogen atoms replaced with deuterium (e.g. CD3CD2OH).
How it works:
- The magnetic field is held constant while a band of frequencies is applied as a pulse to the sample.
- The radio-frequency results in the nuclei energy level increasing; immediately after this increase, there will be a greater than normal amount of protons at the higher energy level. Some of these emit radiation as they move down to the lower energy level.
- This radiation is measured by the detector and sent to the recorder.
- The radiation is weak and the process is over very quickly, so it is repeated many times to ensure that a valid result is returned.
- A graph of absorption against frequency (referred to as chemical shift) is produced.
Interpreting the Spectra
- As afore mentioned, the n.m.r. can distinguish between 1H atoms in different environments.
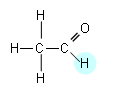
- If we analysed a molecule of ethanal, there would be two different peaks, as there are two different environments (represented by different colours below):
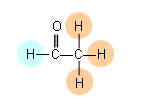
- It will require more energy, thus a higher frequency to move the 1H nucleus attached to the C=O group, as it is within the magnetic field of the oxygen atoms lone pair of electrons. (The chemical shift values can be found in the data booklet).
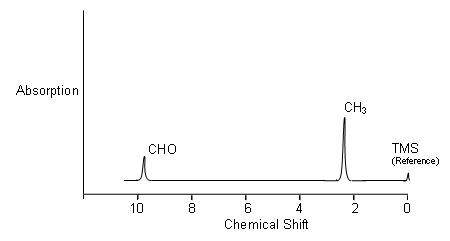
- The n.m.r. spectrum for ethanal is shown below:
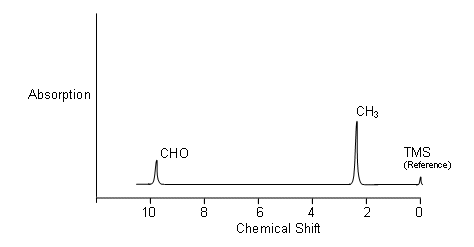
- The area under the peaks is proportional to the number of protons absorbing each time; i.e. there are three times as many protons in the CH3 group than the CHO group, thus the area under the CH3 peak is three times the area under the CHO peak.
- Tetramethylsilane (TMS) is the standard reference (gives a sharp signal well away from the ones of interest to chemists, and is relatively inert) which all the other absorptions are compared with. The extent to which a signal differs from TMS is called the chemical shift.
- NB. On the chemical shift axis, the scale is reversed.
- The n.m.r. machine actually produces a signal which is far more detailed than the ethanal example (above).
High Resolution N.M.R
- High resolution n.m.r spectrographs have much more detail than the low resolution n.m.r explained previously.
- The extra detail arises because the 1H nuclei, which behave like small magnets, can be in one of two orientations depending upon whether they are in a low or high energy level.
- Therefore, some of the 1H nuclei will be spinning with the external field and the others will be spinning against it. The probability of each event is equal.
- The extra detail can provide us with more information about the molecule.
- For example, take for instance a molecule of ethanal:
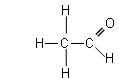
- There are two different proton environments (the CH3 group and the CHO group); the low resolution spectrum would look like the following:
- However, the high resolution graph looks like the following:
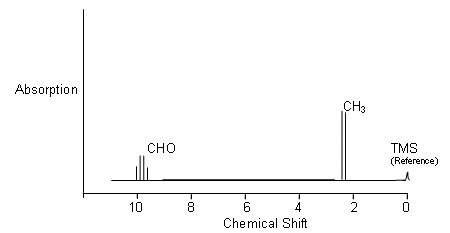
- The CHO peak is split into four peaks (a quartet) and the CH3 group is split into two peaks (a doublet).
- This is due to the effect of the 1H nuclei on the adjacent carbon atom acting like small magnets.
- Looking closer at this idea, the separate peaks can be accounted for.
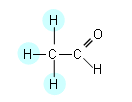
- If we focus first on the 1H nuclei within the CH3 group.
- There are two different peaks for the CH3 group, and these are brought about by the effect of the CHO group.
- As mentioned previously, the hydrogen nuclei have an equal probability of spinning with or against the external field. There is only one 1H nuclei and therefore there are two possible spins:
S-N (aligned with external field)
N-S (aligned against external field)
- Therefore there are two different magnetic effects on the protons within CH3 group when the absorbance is being read; there are two peaks.
- Now looking at the CHO group.
- The four peaks peaks are brought about by the hydrogen nuclei in the CH3 group.
- The arrangements of the spins (against or with external field) can be:

- There are four different arrangements, hence four peaks. The ratio of the heights of the peaks is 1:3:3:1 as there are three times as many arrangements of “2 with, 1 against” and “1 with, 2 against” compared to “all against” and “all with”.
- Therefore counting the number of peaks allows you to work out the number of hydrogens attached to the adjacent carbon atom (if there were 2 peaks, the adjacent carbon would have 3 hydrogen atoms attached to it; if there were n peaks, the adjacent carbon would have n-1 hydrogen atoms attached to it)*
- *When referring to the adjacent carbon- the next hydrogen in a different environment (attached to a carbon).
Example:
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home












