
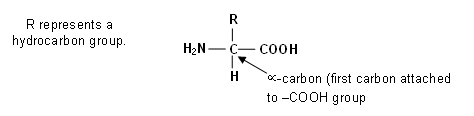
Amino Acids
- Alpha-Amino acids contain both the NH2 (amino) group and the COOH (carboxylic acid) group attached to the same carbon atom.
- Proteins are made from amino acids and so they are very important in living organisms.
- In addition to forming proteins, amino acids also have many other uses, for example, the neurotransmitter GABA (gamma-aminobutanoic acid or 4-aminobutanoic acid).
H2N-CH2-CH2-CH2-COOH
- Amino acids have two functional groups; they are therefore examples of bifunctional compounds.
- Bifunctional compounds often share the same properties of the two functional groups.
- However, this is not the case with amino acids, as both of their functional groups interact.
- The COOH group acts as an acid (donating protons) and the NH2 group acts as a base (accepting protons); therefore these two groups can react with each other forming zwitterions (compounds containing both anionic and cationic groups):
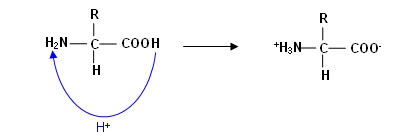
- An aqueous solution of an amino acid is formed mainly of zwitterions (solution is neutral, unless there is an extra COOH or NH2 on the R group); this gives the solution certain characteristics that are common of ionic compounds:
-
They are soluble in water.
- They have a higher melting and boiling point than expected.
- When they dissolve in water, the NH2 group accepts a proton and the COOH group donates one to another water molecule.
H2O + H2NóCH(CH3)óCOOH + H2O  HO- + +H3NóCH(CH2)óCOO- + H3O-
HO- + +H3NóCH(CH2)óCOO- + H3O-
The OH- and H3O+ ions then go on to react with each other, to restore the equilibrium within water:
OH-(aq) + H3O+(aq)  2H2O(l)
2H2O(l)
- This maintains that the overall solution is neutral.
- Zwitterions act as good buffers; addition of small quantities of acid or alkali to the solution has little affect upon the pH of the solution:
OH-(aq) + +H3NóCH(R)óCOO- (aq) 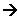 H2O(l)+ H2NóCH(R)-COO-(aq)
H2O(l)+ H2NóCH(R)-COO-(aq)
H3O+(aq) + +H3NóCH(R)óCOO-(aq) 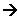 H2O(l) + +H3NóCH(R)-COOH(aq)
H2O(l) + +H3NóCH(R)-COOH(aq)
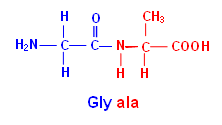
Naming an Amino Acid Sequence
- Amino acid sequences are named in the direction with the free NH2 group on the left; for example:
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home


 HO- + +H3NóCH(CH2)óCOO- + H3O-
HO- + +H3NóCH(CH2)óCOO- + H3O-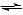 2H2O(l)
2H2O(l)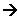 H2O(l)+ H2NóCH(R)-COO-(aq)
H2O(l)+ H2NóCH(R)-COO-(aq)
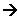 H2O(l) + +H3NóCH(R)-COOH(aq)
H2O(l) + +H3NóCH(R)-COOH(aq) 
