
Properties of Polymers
- The intermolecular forces between polymer chains can have a huge effect upon the properties of the polymer.
-
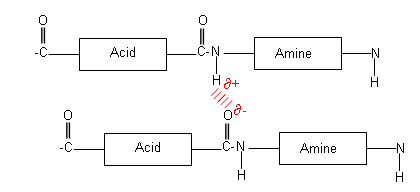
In polyamides, the presence of a the amide linkage allows the polymer chains to hydrogen bond with one another, this prevents the chains from sliding past each other as easily, and so makes them less flexible.
-
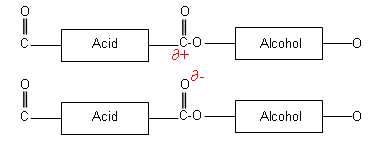
In polyesters, permanent dipole permanent dipole interactions can also occur, which holds the polymer chains together more firmly.
-
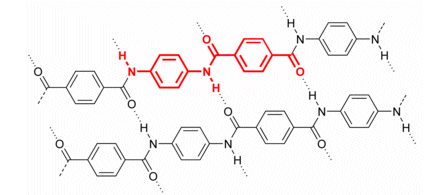
In Kevlar, a combination of the benzene ring (which is hard to rotate) and the presence of hydrogen bonds makes its structure very strong. Kevlar is fireproof, and forms very strong fibres (its fibres are 20 times stronger than the equivalent weight in steel).
The effect of temperature change on polymers
- When substances made of small molecules are heated, they simply melt and form a free-flowing liquid; however with polymers the process is more complex.
- When polymers are cooled, they will often become very brittle. When heated, the polymers will first become flexible before melting.
- This is due to their structure. In some polymers, there are areas of crystalline and amorphous regions. In the “glassy” state, the tangled polymer chains in the amorphous region become “frozen”, which prevents easy movement of the chains relative to each other. If the polymer is forced to change shape, it does so by breaking.
- When the glassy material is heated, the polymer chains will reach a temperature at which they can move relative to each other (the glass transition temperature Tg). On further heating of the glassy polymer, it becomes flexible and shows the more conventional properties expected of a plastic.
- Eventually, if the polymer is heated enough, the melting temperature Tm will be reached (the plastic becomes a viscous fluid).
-
This process is reversible for thermoplastics.
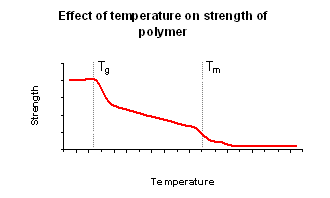
- Today, polymers are designed to have Tg and Tm values which are related to the desired properties of the substance.
- Flexibility can occur when the chains are not tightly packed and some freedom of movement is possible (bulky side-groups, for example, may reduce the flexibility of a polymer, as they prevent the chains sliding past each other).
- Bulky side-groups also affect the amount of rotation that can occur around the linking bonds.
- Intermolecular forces may also have their effect upon flexibility; stronger intermolecular forces will mean that the chains are held together more tightly and are therefore less able to move relative to each other.
Designing Polymers
- When looking for a substance for a particular application, it is sometimes possible that there will not be a polymer available that will have all the characteristics that are required.
- Techniques such as co-polymerisation, the use of plasticizers and the possibility of producing polymer alloys can make a polymer more suitable to its application.
Co-polymerisation
- This involves incorporating two different monomers into a polymer chain during the polymerisation process.
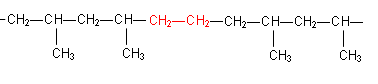
- For example, polypropene has a Tg of around -10oC; for some purposes a lower Tg would be more suitable. One way to lower the Tg of polypropene is to add ethene to the polymer during the polymerisation process; co-polymerisation.
-
A section of the chain would be represented as:
Plasticisers
-
Plasticisers can be used to make polymers more flexible; they are basically a molecular lubricant that allows the polymer chains to slide past each other easier.
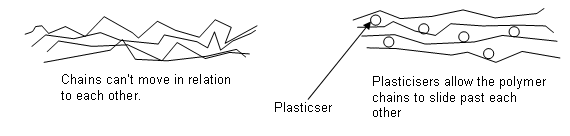
- Plasticisers are carefully selected so that they are compatible with the polymer they are used with. Di-(2-ethylhexyl)adodate (DEHA) is commonly used as a plasticiser for PVC; however there is growing concern that plasticizers added to PVC film used for wrapping food can migrate into fatty food and may be harmful to health.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry