
Esters
- Esters are the functional group isomers of carboxylic acids.
-
Esters are formed when a molecule of an organic acid reacts with an alcohol.
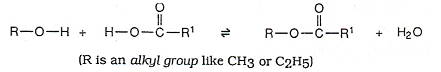
- The above reaction is a condensation reaction, as two small molecules have joined together to form a larger molecule with the elimination of a smaller molecule (water in the reaction above).
- An acid catalyst is usually present in the above reaction, as the esterfication process would be too slow without it.
-
Esters have strong, sweet smells. They are used widely in glues, as solvents, and in fragrances.

-
Esters are named from the alcohols and acids from which they are derived. For example,
Ethanol and Ethanoic acid react to produce Ethyl Ethanoate 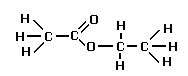
Making Esters
- There are three methods for making esters:
1) Esterification:
2) Acylation using an acid chloride:
- The reaction of an acid and an alcohol to produce the ester and water. Requires a concentrated strong acid catalyst, such as 1 mol dm-3 H2SO4, and needs to be heated under reflux to ensure the reaction reaches equilibrium:
CH3COOH(aq) + CH3OH(aq)CH3COOCH3(aq) + H2O(l)
3) Acylation using an acid anhydride:
- Acid chlorides are very reactive and fume in moist air forming hydrogen chloride. They react at room temperatures with alcohols/phenols to form esters.
CH3COCl(aq) + CH3CH2CH2OH (aq)CH3COOCH2CH2CH3(aq) + HCl (aq)
- Less reactive than using an acid chloride (but more reactive than using carboxylic acids) and are easier to handle. They react, when warmed, with alcohols/phenols to produce esters.
(CH3CO)2O(aq) + CH3CH2CH2OH (aq)CH3COOCH2CH2CH3(aq) + CH3COOH (aq)
Hydrolysis of Esters
- The reverse reaction of an esterfication reaction is a hydrolysis reaction.
- Esters are reasonably unreactive compounds (used as solvents for this reason). They can be hydrolysed using acidic or alkaline conditions.
Acidic Hydrolysis
-
The conditions for this reaction are a moderately concentrated strong acid (catalyst) mixed with the ester, heated under reflux. After equilibrium is reached, no further hydrolysis takes place:
CH3COOCH3(aq) + H2O (l)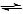 CH3COOH (aq) + CH3OH (aq)
CH3COOH (aq) + CH3OH (aq)
Alkaline Hydrolysis
-
Sometimes referred to as saponification, this reaction uses dilute alkaline solution and reflux:
CH3COOC6H5(aq) + NaOH (aq)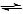 CH3COO-Na+ + C6H5OH (aq)
CH3COO-Na+ + C6H5OH (aq)
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry