
Entropy and Equilibrium
- According to the Second Law of Thermodynamics, a change takes place in the direction which corresponds to an increase in
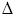 Stotal.
Stotal.
- This is the case for the freezing of water below temperatures of 0oC; however, above this temperature the
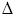 Stotal for the freezing of ice is negative and so the reverse occurs; the ice melts.
Stotal for the freezing of ice is negative and so the reverse occurs; the ice melts.
- When the
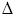 Stotal is zero, there will be no tendency for the reaction to go in either direction; it will be at equilibrium.
Stotal is zero, there will be no tendency for the reaction to go in either direction; it will be at equilibrium.
- This can be shown once again for the freezing and melting of water:
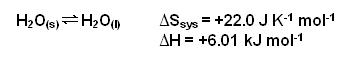
- At 273K, the
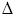 Stotal for the equilibrium reaction is:
Stotal for the equilibrium reaction is:
- The reaction is at equilibrium; liquid water and ice exist together at 0oC.
At equilibrium, the 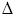 Stotal is equal to zero.
Stotal is equal to zero.
- Even though theoretically the equilibrium occurs at 0oC, the temperature does not have to be exactly equal to this for it to occur.
- If the conditions happen to be standard, then the standard entropy change will be zero; however, the change rarely is zero at equilibrium.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home
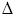 Stotal.
Stotal.
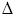 Stotal for the freezing of ice is negative and so the reverse occurs; the ice melts.
Stotal for the freezing of ice is negative and so the reverse occurs; the ice melts.
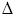 Stotal is zero, there will be no tendency for the reaction to go in either direction; it will be at equilibrium.
Stotal is zero, there will be no tendency for the reaction to go in either direction; it will be at equilibrium.
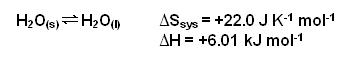
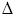 Stotal for the equilibrium reaction is:
Stotal for the equilibrium reaction is:
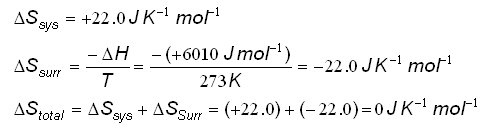
 Stotal is equal to zero.
Stotal is equal to zero. 