
Storing Carbon Dioxide
- The solubility of gases decreases as temperature increases; this is illustrated in the following graph which shows the solubility of carbon dioxide in water:
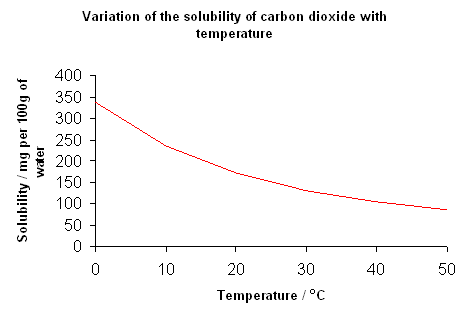
- There is also a relationship between pressure and the solubility of gas; the higher the pressure, the greater the volume of gas dissolves in the solution.
- Carbon dioxide is put into drinks under considerable pressure in order to make them fizzy.
- Therefore a high pressure and low temperature will help keep your can of coke fizzy.
- These conditions also favour carbon dioxide dissolving in the oceans, thus removing it from the atmosphere, helping to maintain a constant environment on Earth.
- The exchange of CO2 between the atmosphere and the ocean takes place rapidly; much faster than if water was left to rest in a glass.
- The uptake of CO2 by the oceans is made much more efficient by the effects of marine life; for example, CO2 is taken up by phytoplankton (for photosynthesis), the phytoplankton is then eaten and metabolised by animals which release CO2 into the water.
- Carbon dioxide is much more polar than O2 and N2; this is related to the fact that it has polar bonds which can interact with the dipolar water molecules.
- At the surface of the oceans, the following equilibrium is taking place:
CO2 (g) CO2 (aq)
CO2 (aq)
- Some of the carbon dioxide then reacts with water:
CO2(aq) + H2O(l)  H+(aq) + HCO3-(aq)
H+(aq) + HCO3-(aq)
HCO3-(aq)  H+(aq) + CO32-(aq)
H+(aq) + CO32-(aq)
- This removes some of the aqueous carbon dioxide from the water, and thus shifts the position of equilibrium (in the reaction of CO2 dissolving in water) to the right.
- Much of the carbon dioxide we pump into the atmosphere is absorbed by the oceans in this way (probably around 35-50% is removed in this way).
- The aborption of CO2 into the oceans is a continual process; the surface water that is rich in CO2 sinks to the deeps of the oceans, where it is stored for hundreds of years.
Dissolving calcium carbonate
The overall equations listed previously can be combined into one equation:
CO2(aq) + H2O(l)  2H+(aq) + CO32- (aq)
2H+(aq) + CO32- (aq)
- According to Le Chatelierís principle, removing the H+ ions will result in more CO2 dissolving into the ocean.
- One way to remove H+ ions would be to add a base; however this is not a very practical proposition where the oceans are concerned.
- There are, however, many marine organisms that are able to remove CO32- ions from sea water; they use the ions to form protective shells of calcium carbonate; CaCO3.
- Therefore by forming calcium carbonate shells, the small marine organisms are doing their part to combat the increasing levels of carbon dioxide in the atmosphere.
- Billions of years ago, the atmosphere contained much more carbon dioxide; as a result of this, shell production flourished.
- Limestone cliffs are the remains of the calcium carbonate shells formed by the marine organisms billions of years ago.
- Calcium carbonate is insoluble in salt water at the surface of the oceans, and is therefore a useful product for making protective shells.
- However, calcium carbonate does slightly dissolve in pure water:
CaCO3(s) + aq  Ca2+(aq) + CO32- (aq)
Ca2+(aq) + CO32- (aq)
- The solubility product for calcium carbonate is 5 x 10-9 mol2 dm-6.
- When the Ca2+ ions and CO32- ions mix in solution, one of three things can occur:
- Calcium carbonate precipitates out of the solution; this occurs when the value of [Ca2+] x [CO32-] is greater than the solubility product.
- The dissolved ions are in equilibrium with the calcium carbonate; this occurs when [Ca2+] x [CO32-] is equal to the solubility product.
- The ions remain in solution; this occurs when [Ca2+] x [CO32-] is less than the solubility product.
- At the surface of the oceans, the Ca2+ and CO32- ions are at high concentrations, and so the calcium carbonate is insoluble.
- However, deeper down in the ocean, the pressure is higher and the temperature is lower; the Ksp is greater under these conditions and calcium carbonate is therefore more soluble.
- So as the calcium carbonate moves deeper down the oceans, it begins to dissolve, forming a marine snow (lots of remains of dead marine creatures).
- Any organic material that reaches the lower regions of the ocean is then consumed by other organism, which release CO2.
- The extra CO2 promotes the dissolving of calcium carbonate even further.
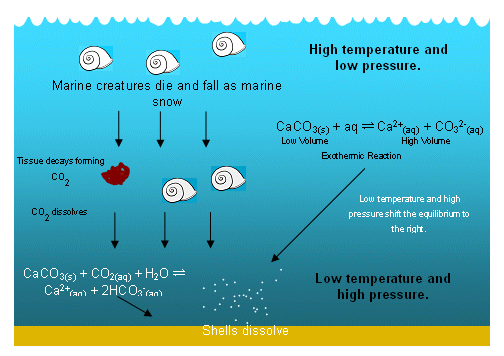
- There are no shells on the ocean floor; they have all dissolved by the time they reach the low temperatures and high pressures.
- This means that the calcium carbonate cliffs must have formed in shallow seas; it is likely that the water was also warm and tropical.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home

 CO2 (aq)
CO2 (aq) H+(aq) + HCO3-(aq)
H+(aq) + HCO3-(aq) H+(aq) + CO32-(aq)
H+(aq) + CO32-(aq)

 CO2 (aq)
CO2 (aq) H+(aq) + HCO3-(aq)
H+(aq) + HCO3-(aq) H+(aq) + CO32-(aq)
H+(aq) + CO32-(aq) 2H+(aq) + CO32- (aq)
2H+(aq) + CO32- (aq) Ca2+(aq) + CO32- (aq)
Ca2+(aq) + CO32- (aq)