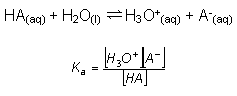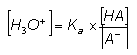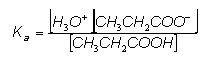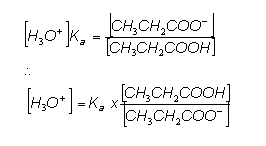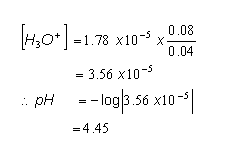Buffer Solutions
-
A buffer is a solution in which the pH resists change when a small amount of strong acid or base is added.
- Buffers consist of either a weak acid and salt (acid buffer), or a weak base and salt (a base buffer).
- Acid buffers maintain pH’s on the acid side of neutrality (<7); base buffers maintain the pH on the basic side of neutrality.
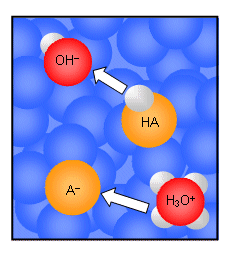
- The action of buffer solutions can be understood by looking at the dynamic equilibrium that occurs between a weak acid and its conjugate base in water:
HA(aq) + H2O(l)  H3O+(aq) + A-(aq)
H3O+(aq) + A-(aq)
- If a strong acid is added to the solution, the position of equilibrium will shift to oppose the change; the position of equilibrium will move to the left. The newly formed H3O+ ions will transfer protons to the A- ions forming the weak acid and H2O molecules.
- Because the added H+ ions are removed by the solution by the A- ions, the pH of the solution remains almost unchanged; the A- ions have acted as a sink for the H+ ions.
- If a strong base is added to the solution, then the incoming OH- ions will remove protons from the HA molecules to form H2O and A- ions. In this case, the HA has acted as a source of H+ ions; consequently the pH of the solution remains unchanged.
- The same occurs with base buffers; if strong acid is added, then the basic salt, NH3 for example, will accept protons from the acid becoming NH4+.
- If strong base is added, then the base, NH4+, will donate protons to it, becoming NH3.
- A number of assumptions must be made when considering bases:
- All the A- ions come from the salt.
The weak acid, HA, supplies very few A- ions to the solution in comparison to the fully ionised salt.
- Almost all of the HA molecules put into the buffer remain unchanged.
If the HA molecules dissociate then the pH will change; however, very few of them do as the position of equilibrium for the dissociation of the weak acid is far to the left, due to presence of H+ and A- ions.
Calculations involving buffers
- In order to perform calculations on buffer solutions, all is needed is the Ka expression for the relevant weak acid:
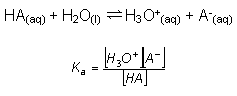
- Rearranging this gives:
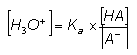
- The value of the [H3O+], and therefore the pH of the solution depends on:
- The value of Ka
This provides the course tuning for the buffer solution; Ka values are usually within the pH range of 4-10. Selection of a particular weak acid determined the region of the pH range the buffer is in.
- The ratio of [salt]:[acid]
The provides the fine tuning for the pH of the buffer. Changing the ration from around 3:1 to 1:3 changes the [H+] by a factor of approximately 9, and therefore changes the value of the pH by 1 unit. The ratio should not be too far outside this range, otherwise there will not be enough acid or salt.
- The buffer is not affected by dilution, as when water is added, both the concentration of the weak acid and the salt change by the same amount; therefore their ratio of concentrations remains unchanged.
Example Calculation
- A buffer solution consisting of 0.040 mol dm-3 CH3COO-Na+(aq) and 0.08 mol dm-3 CH3COOH(aq) is prepared at 1 atm, 298 K. Calculate the pH of the buffer solution.
- We first need to begin by identifying the weak acid and its conjugate base; we then need to write the equilibrium between the two:
CH3COOH(aq)  CH3COO-(aq) + H+(aq)
CH3COO-(aq) + H+(aq)
- This is a reaction at dynamic equilibrium and so an Ka expression can be written:
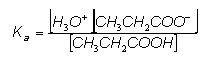
- We can rearrange the equation to give:
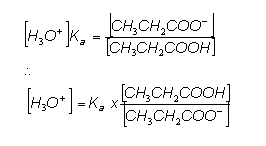
- From the data book, the Ka value for ethanoic acid is 1.78 x 10-5, so:
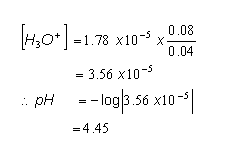
Use of Buffers
- Buffer solutions are present in many living systems, where reactions must take place under narrow pH ranges; for example our blood is buffered to pH 7.35-7.45, our saliva to pH 6.4-6.8 and our stomach juices to pH 1.6-1.8.
- Carbonic acid in the oceans acts as a buffer solution:
In low pH’s CO32- “mops up” the excess H+ ions:
CO32-(aq) + H+ (aq)  HCO3- (aq)
HCO3- (aq)
At high pH’s the reverse of the reaction occurs:
HCO3-(aq)  CO32-(aq) + H+(aq)
CO32-(aq) + H+(aq)
- Many chemical processes also require buffer solutions; dyeing fabrics can be ineffective at the wrong pH values.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home
 H3O+(aq) + A-(aq)
H3O+(aq) + A-(aq)