
Ultraviolet and Visible Spectroscopy
Coloured Compounds
- Substances appear coloured when they absorb radiation from the visible region of the electromagnetic spectrum (400-700 nm); the light that transmitted or reflected will be lacking in certain frequencies of visible light and so will appear coloured.
- Carrots contain the pigment carotene:
- Carotene absorbs blue light strongly, so the blue light is lacking when it reaches our eyes; therefore carrots appear orange-red.
- A spectrometer can be used to measure the quantity of light absorbed by the pigment at each wavelength.
- The recorder plots out the intensity of absorption against the wavelength. The absorption spectrum for carotene in hexane is shown below:
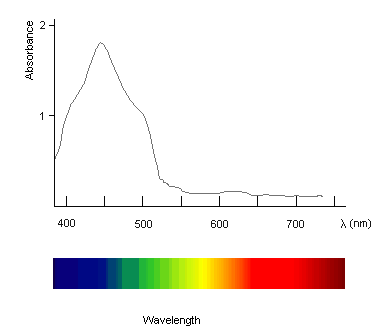
Absorption Spectra
- The ultra-violet and visible spectrometer works in a similar way to an infrared spectrometer (What’s in a Medicine).
- Ultraviolet or visible radiation from a light source is split into two beams.
- One of the beams goes through the sample, and the other goes through a reference chamber, containing only the pure solvent.
- The beams are sent along the same path, in separate alternating pulses, achieved using a beam chopper (rotating disc with segment cut out of it).
- The beams are then compared to give the absorption spectrum of the sample.
- Our eyes are unable to detect ultraviolet radiation; if a substance absorbs in this region, it does not affect its colour.
- Therefore, substances, such as benzene, which only absorb in the ultraviolet region of the spectrum, appear colourless:
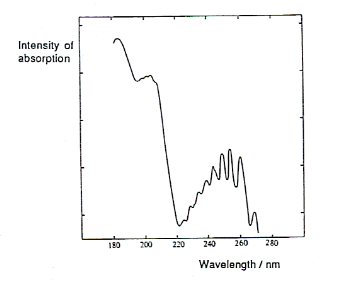
- The wavelength is plotted on the horizontal axis and the intensity of the absorption is plotted on the vertical axis.
Interpreting Visible and UV Spectrums
- There are three main features of a spectrum that are of interest:
- The wavelength of the radiation absorbed.
- The intensity of the absorption.
- The shape of the absorption band.
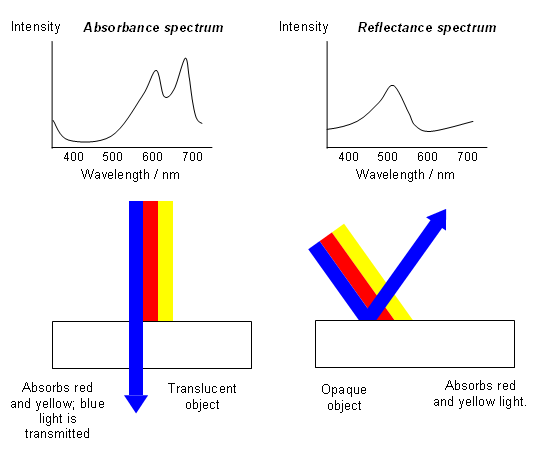
- When analysing spectra, chemists often give the wavelength of the maximum absorption,
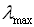 . For carotene, the
. For carotene, the 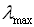 occurs in the blue region of the spectrum; it is red/orange in colour, which is the complimentary colour to blue.
occurs in the blue region of the spectrum; it is red/orange in colour, which is the complimentary colour to blue.
- The intensity of the absorption is related to the concentration of the solution and how far the light must travel through it.
- Standard molar values are quoted so that values of different compounds can be compared.
- The intensity of the absorption is important, as it determines the amount of pigment/dye needed to produce a good colour.
- The shape and width of the absorption band is important in governing the shade and purity of the colour seen.
Reflectance Spectra
- In some cases, it is difficult to make a solution of a coloured substance to record its spectrum; in cases like this, chemists can use reflectance spectroscopy.
- A light source is shone onto the sample and the composition of the reflected light is examined; this is the part of the light that has not been absorbed by the pigments. It is therefore like the negative of the absorption spectrum.
Electronic changes
- When visible light falls on a coloured compound, certain frequencies of it are used to promote electrons to a higher energy level.
- The electron does not stay promoted for very long; the energy absorbed is re-emitted.
- It is not necessarily re-emitted all at the same time; the molecule may emit a smaller quantum of energy and only fall back to an intermediate energy level; the remaining energy can then be converted to kinetic energy for the molecules, which leads to them moving around faster and becoming warmer.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home






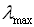 . For carotene, the
. For carotene, the 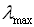 occurs in the blue region of the spectrum; it is red/orange in colour, which is the complimentary colour to blue.
occurs in the blue region of the spectrum; it is red/orange in colour, which is the complimentary colour to blue.
