
Reactions of The Arenes
- The six electrons in benzene's delocalised system do not belong to any one carbon and are free to move around the ring.
- They provide benzene with a high electron density.
- Regions of high density tend to attract positive ions, or atoms with a partial positive charge; benzene, like the alkenes reacts with electrophiles.
- The reactions are much slower with benzene, due to the high energy required to disrupt the delocalised electron system.
- Where alkenes undergo electrophilic addition reactions with electrophiles, benzene undergoes electrophilic substitution reactions; this is to maintain the stable benzene ring system in the product.
- There are many electrophilic substitution reactions involving benzene; however the mechanism is similar for each one and can be summarised as follows (X represents an electrophile):
- The electrophile is attracted to delocalised electrons and the benzene ring donates a pair of electrons to it forming a covalent bond.
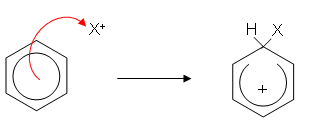
- The ring is now only partially delocalised; it has two less electrons and so has a positive charge. The hydrogen gives up its electrons to the benzene ring, reforming the delocalisation.
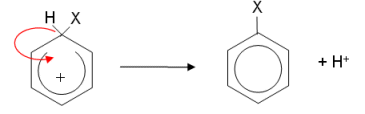
- What makes the electrophilic substitution reactions different is how the electrophiles are first formed.
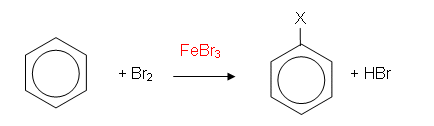
Bromination of Benzene
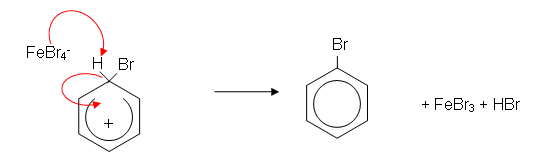
- In the presence of iron filings or iron(III) bromide, the bromine is decolourised and fumes of hydrogen bromide (HBr) are given off. The reaction which takes place between bromine and benzene is a substitution reaction:
- The mechanism works as follows:
- The benzene ring induces a dipole in the Br2 molecule. The slightly positively charged end of the bromine molecule is now slightly electrophilic. The iron filings help speed up the reaction by reacting with bromine to form iron(iii) bromide; this then accepts a pair of electrons from the slightly negative end of the polarised bromine molecule, forming a more reactive electrophile:
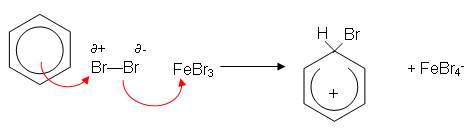
- A H+ ion is lost from the ring; this reacts with FeBr4- to produce HBr, regenerating the catalyst:
Nitration of Benzene
- An NO2 group won’t react directly with benzene; it must have a positive charge to do so.
- Therefore nitric acid is reacted with sulphuric acid to form the nitronium ion, NO2+.
HNO3(aq) + 2H2SO4(aq) 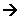 NO2+(aq) + HSO4- (aq) + H3O+ (aq)
NO2+(aq) + HSO4- (aq) + H3O+ (aq)
- If the reaction mixture is kept below 55oC, then the product is nitrobenzene; at higher temperatures, further substitution of the ring can occur forming di- and tri- substituted compounds.
- The mechanism for the nitration of benzene works as follows:
- NO2+ is attracted to the delocalised electrons in benzene. The benzene ring donates a pair of electrons to it forming a covalent bond:
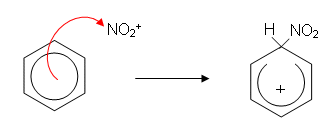
- The ring is now only partially delocalised; it has two less electrons and so has a positive charge. This positive charge attracts the hydrogen sulphate molecule which donates a pair of electrons to the hydrogen atom. The hydrogen gives up its electrons to the benzene ring, reforming the delocalisation. This reforms the sulphuric acid which has acted as a catalyst:
Sulphonation of Benzene
- This is useful for making benzene soluble in water. The mechanism takes place when heated under reflux with concentrated sulphuric acid.
There is a reversible reaction for the dissociation of H2SO4:
H2SO4(aq) 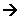 H2O (l) + SO3(aq)
H2O (l) + SO3(aq)
- The SO3 can undergo an electrophilic substitution reaction with benzene:
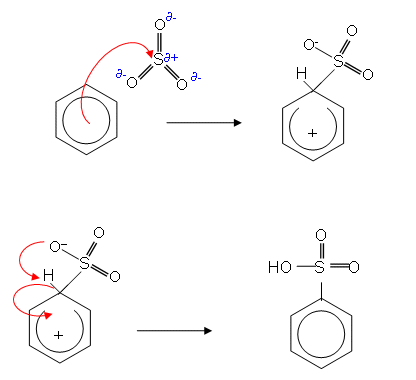
- The product is a strong acid which forms salts in an alkaline solution:
- Most solid detergents contain salts of this kind; a long alkyl group attached to a benzene ring.
- The hydrocarbon part of the molecule mixes with fats and the ionic part mixes with water.
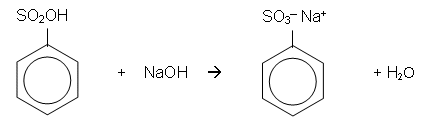
Chlorination of Benzene
- It is possible for a chlorine atom to be substituted onto a benzene ring in much the same way as a bromine atom can be; however, the catalyst for the chlorination of benzene is usually aluminium chloride (AlCl3).
- Aluminium chloride helps to polarise the chlorine molecule.
- The reaction must be carried out under anhydrous conditions (without water) as aluminium chloride reacts vigorously with water.
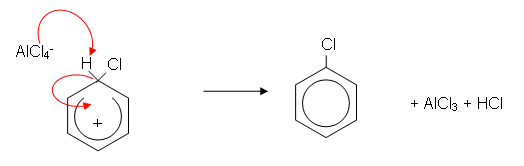
- The mechanism for the reaction is as follows:
- The benzene ring induces a dipole in the Cl2 molecule. The slightly positively charged chlorine atom donates a pair of electrons to the AlCl3 molecule forming a covalent bond. The benzene ring then donates a pair of electrons to the positive chlorine atom forming a covalent bond:
- The AlCl4- then donates a pair of electrons to the hydrogen atom, which donates a pair of electrons to the benzene ring, reforming the delocalisation. AlCl3 is also reformed and HCl is produced:
Friedel-Crafts reactions
- Aluminium chloride can also act as a catalyst to help polarise halogen-containing organic molecules and cause them to substitute in a benzene ring.
- Once again, this reaction needs to be carried out under anhydrous conditions, as aluminium chloride reacts vigorously with water.
- Aluminium chloride reacts with either the acyl chloride or halegenoalkane:
AlCl3(aq) + CH3COCl (aq) 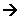 AlCl4-(aq) + CH3CO+
AlCl4-(aq) + CH3CO+
- The mechanism then goes as follows:
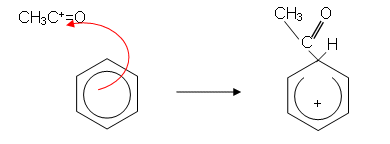
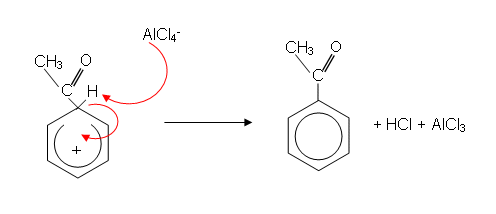
- Because an alkyl group has been introduced into the ring, the reaction is sometimes called alkylation.
- A similar reaction also takes place when benzene is reacted with a halogenoalkane or an acid anhydride:
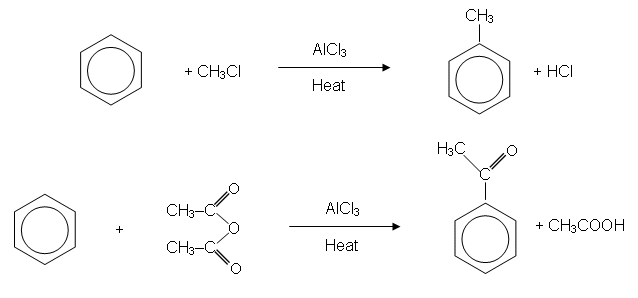
Summary
-
Benzene is very important for the synthesis of dyes, pharmaceuticals and perfumes etc.
- Electrophilic substitution reactions provide us with ways of introducing different groups into the benzene ring.
- These groups can then be modified further and more complex molecules can be synthesised.
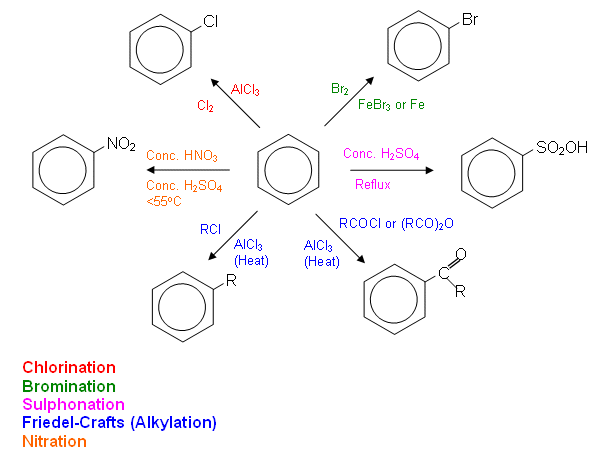
- The conditions for the important reactions are summarised in the diagram below:
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home
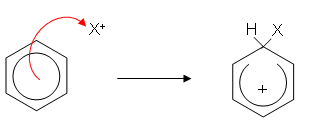



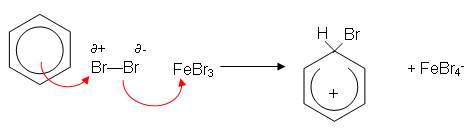

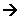 NO2+(aq) + HSO4- (aq) + H3O+ (aq)
NO2+(aq) + HSO4- (aq) + H3O+ (aq)









