
How dyes attach themselves to fabric
- There are both natural fibres and synthetic fibres; due to the different functional groups on the fibres, different dyes are required to colour them.
- There are a number of different types of dye:
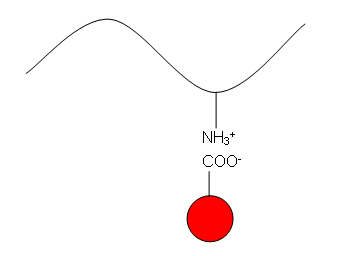
Acidic Dyes
- These contain acid groups, such as –COOH and –SO3H which form attractions to the slightly basic –NH groups in the amide links of wool, silk and nylon:
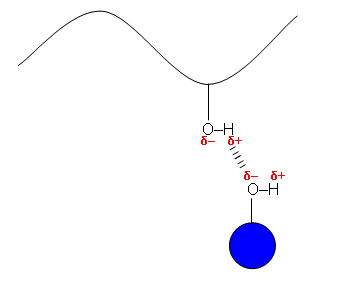
Direct Dyes
- These bond to fabrics by hydrogen bonding and so are particularly attracted to cellulose fibres, such as cotton and rayon, which have many –OH groups.
- Hydrogen bonds are weak compared to covalent bonds and so the dyes are only fast if the molecules are long and straight; they must be able to line up with the cellulose fibres and form several hydrogen bonds.
Disperse/vat dyes
-
These do not dissolve in water, but instead are oxidised in the solution and physically held in place within the fibres.
- Many azo compounds are examples of vat dyes.
Fabric reactive dyes
- Fastness is a measure of how strongly a dye is attached to a fabric and is an important indication as to whether the dye will move into water when the material is washed.
- For many years, chemists dreamed of developing fast dyes that would covalently bond to fabrics rather than only joining to the fabric by weak intermolecular forces.
- During the 1950’s, a group of chemists working for ICI embarked on their search for a better dye for wool.
- William Stephen, a member of that group, decided to modify the structure of azo dyes by adding reactive groups in the hope that they would combine with the amino groups of proteins in wool.
- One of his ideas was to modify an azo dye containing an amino group by reacting it with trichlorotriazane:
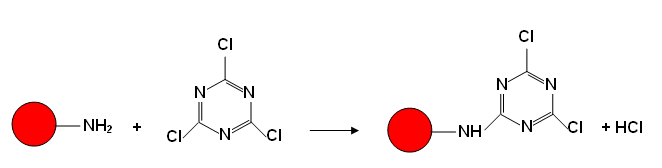
-
It was hoped that the new dye would react with wool:
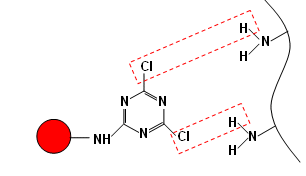
- However, the results were very poor and so more work needed to be done on the dyes.
- Stephen realised that the reaction would be more likely to happen in alkaline conditions; however, this caused a problem, as alkaline conditions would damage the wool.
- Instead, they used the dyes with cotton, which would not be damaged by the alkaline conditions.
- This was a success; the dye molecules reacted with both the amine and hydroxyl groups on the cotton fibres. The first fibre reactive dyes had been produced.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home





