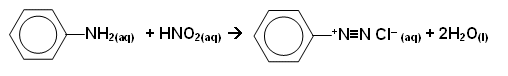Azo Compounds
- Azo compounds contain the –N=N– group:
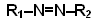
- In aromatic azo compounds, the R groups are arene rings; the structures of these are more stable than if the R groups are alkyl groups.
- This is because the –N=N– group becomes part of an extended delocalised system involving the arene groups.
- The aromatic azo groups are highly coloured and are often used as dyes.
- Aromatic azo compounds are formed by a coupling reaction between a diazonium salt and a coupling agent.
Diazonium Salts
- The diazonium salts are very unstable; the only relatively stable diazonium salts are the aromatic ones, and these are not particularly stable.
- This is because the presence of the benzene ring with its high electron density stabilises the –+
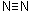 group.
group.
- Benzenediazonium chloride is an example of a diazonium salt:
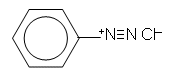
- In aqueous solution, Benzenediazonium chloride decomposes above temperatures of 5oC and the solid compound is explosive; for this reason, diazonium salts are prepared in ice-cold solutions and are used immediately.
- They are synthesised in a diazotisation reaction- a cold solution of sodium nitrate is added to a solution of arylamine in concentrated acid (below 5oC).
- The acid firstly reacts with the sodium nitrate to form an unstable nitrous acid (nitric(iii) acid):
NaNO2(aq) + HCl(aq) 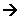 HNO2(aq) + NaCl(aq)
HNO2(aq) + NaCl(aq)
- The nitrous acid then reacts with the arylamine:
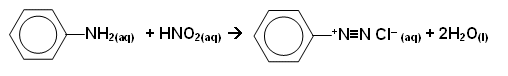
Diazo coupling reactions
- In a diazo coupling reaction, the doazonium salt reacts with another arene (the coupling agent).
- The diazonium salt acts as an electrophile, reacting with the benzene ring of the coupling agent.
- When the ice-cold solution of the diazonium salt is added to a solution containing the coupling agent, a coloured precipitate of an azo compound is formed; many of these compounds are dyes.
- The coupling agent always reacts in the two or four position of the benzene ring (where one position is the functional group).
- The colour of the compound formed depends on the coupling agent that is being reacted with diazonium salt.
Coupling with phenols
- When the diazonium salt is reacted with a phenol, a yellow/orange azo compound is formed:
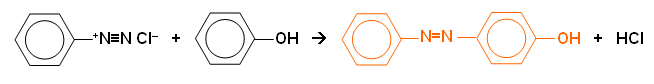
- With an alkaline solution of napthalein-2-ol, a red azo compound is formed:
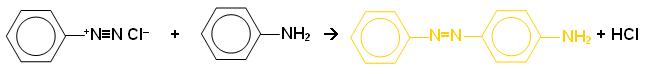
Coupling with amines
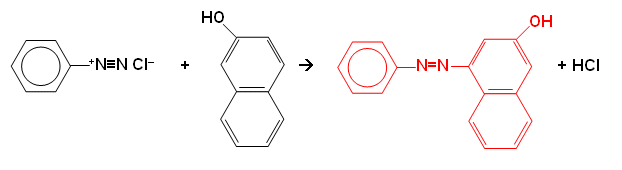
- A yellow dye is often formed when a diazonium salt is reacted with arylamines:
- Many different azo compounds can be formed by coupling different diazonium salts with a range of coupling agents.
- The azo compounds are stable and so their colours do not fade.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home
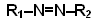

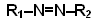
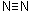 group.
group.
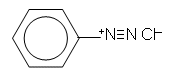
 HNO2(aq) + NaCl(aq)
HNO2(aq) + NaCl(aq)